Prospective, Real-Life Evaluation of a Low-Angle Arterial Entry Closure Device for Endovascular, Outpatient-Based Interventions Using the Arstasis AXERA® 2 System (AXCELERATE Trial)
Eduardo Espinoza, Courtney Harris, Napoleon Cieza-Rubio, John P Pacanowski and Luis R Leon
DOI10.21767/2573-4482.20.05.6
1Department of Vascular and Endovascular Surgery at University of Arizona Banner Medical Center, Tucson, Arizona, USA
2Department of Vascular and Endovascular Surgery at Pima Vascular, Tucson, Arizona, USA
- *Corresponding Author:
- Leon LR Jr
Department of Vascular and Endovascular Surgery at Pima Vascular
Tucson, Arizona, USA
Tel: +1-708-651-4242
E-mail: lleon@pimavascular.com
Received Date: November 21, 2019; Accepted Date: May 04, 2020; Published Date: May 11, 2020
Citation: Espinoza E, Harris C, Cieza-Rubio N, P Pacanowski JP, Leon LR (2020) Prospective, Real-Life Evaluation of a Low-Angle Arterial Entry Closure Device for Endovascular, Outpatient-Based Interventions Using the Arstasis AXERA® 2 System (AXCELERATE trial). J Vasc Endovasc Therapy Vol.5 No.2:6
Abstract
Background: The purpose of the AXCELERATE study was to evaluate the safety and efficacy of the AXERA® 2 vascular closure device in patients undergoing endovascular therapeutic arterial interventions.
Methods: A single-center, single-operator, prospective observational study was performed over 15-months in consecutive patients undergoing outpatient-based, endovascular procedures using the AXERA® 2 device. Diagnostic procedures only were excluded. The primary endpoints were hemostasis success, time to hemostasis (TTH), early (< 60 minutes) sit up time (TTS), time to ambulation (TTA), time to discharge (TTD) and access-related complications.
Results: A total of 105 arterial consecutive procedures were included. The mean age was 73.8 years (55.2% males). The vast majority of procedures were performed by using 6- and 7-French sheaths (48.6% each) and mostly due to ischemic rest pain (35.2%). Heparin was given in all cases and 85.7% were taking an antiplatelet or anticoagulant agent at procedural time. The median TTH was 14.8 minutes of manual compression. Early sit up was accomplished in 80.2% of patients with no complications. The average TTA was 2.4 hours and the TTD was 3.8 hours. A total of 97.1% of patients were discharged without access-related problems and in good clinical condition upon procedural completion. Fourteen complications were seen (13 patients), all of them hematomas > 6-cm (13.3%). Four patients required hospital admission after their interventions (3.8%), 2 required blood transfusions (1.9%) and 2 developed pseudoaneurysms (1.9%). No infectious, vascular thrombotic or other access-related complications were seen during a median follow-up of 117 days. When complications were analyzed with respect to the quartile of occurrence, no statistical differences where found (p>0.05 for all quartiles).
Conclusions: The AXCELERATE trial shows that the use of the AXERA® 2 device reduces TTS, TTA and TTD, while being a safe and effective method of hemostasis for outpatient-based interventions.
Keywords
Endovascular; Arterial interventions; AXCELERATE
Introduction
A shift toward less invasive approaches to treating vascular disease has occurred since the 1990s, with a spectacular increase in endovascular interventions by more than 1,000% in the last 2 decades [1,2]. This shift toward minimally invasive techniques for vascular reconstruction has also caused in the United States (US) an upsurge of Office-Based Vascular Interventional Laboratories (OBL) or outpatient interventional suites [3].
Groin hemostasis after endovascular interventions has a significant impact on patient recovery. Success of hemostasis acquires even more importance in the OBL setting, where the ultimate condition is that the patient will be discharged a few hours after procedural termination. MP has been widely used for hemostasis, but it requires considerable time, resources, prolonged bed rest and patient discomfort [4]. The literature reports control rates of 17 minutes for average time to hemostasis (TTH) with MP and 4.75 hours for average time to ambulation (TTA) in patients undergoing diagnostic interventions [5-9]. Closure devices were designed as a response to high access bleeding rates with percutaneous interventions [4,10-12]. These devices showed efficacy in reducing TTA and patient discomfort but also a new complication set, such as infection, thrombosis, embolism and others [4,11,13-15]. During the hasty endovascular revolution, insufficient outcome analyses have been performed and the performance of several of these new devices remain unproven. Access-site complications continue to be an important cause of morbidity following endovascular procedures [4].
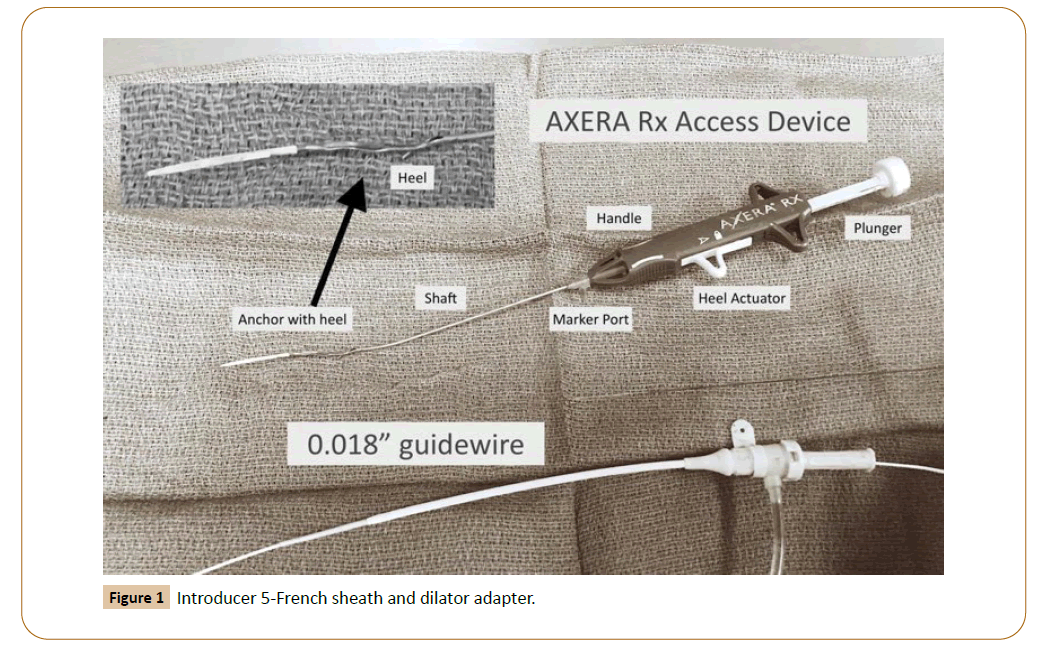
The AXERA® 2 system (Arstasis, Redwood City, California) was cleared by the US Food and Drug Administration in April of 2011 for use in diagnostic catheterization through the CFA approach using 5- or 6-French sheaths (Figure 1). As with any other emergent technology, interventionists continue to test their limits by expanding indications for their use. The AXCELERATE study was designed to evaluate the efficacy and safety of the AXERA® 2 system in patients undergoing endovascular therapeutic arterial interventions in our OBL, in a “real-life” patient scenario by using standard size sheaths and under systemic anticoagulation.
Patients and Methods
Consecutive patients who underwent elective, percutaneous endovascular arterial interventions using the AXERA 2® system at Pima Vascular™ (privately owned OBL, Tucson, Arizona) between December 2014 and March 2016 were prospectively enrolled into the AXCELERATE study. This was an observational study and hence, Institutional Review Board approval and informed patient consent were both waived. Arterial access was gained in all cases by a single endovascular surgeon (LRL) with significant experience with a variety of closure devices. This surgeon served as the Principal Investigator (PI) for the study. In addition, proper extensive training was provided as far as the details of the device and its method for proper deployment by company representatives prior to the beginning of the study, both in vivo and in vitro. Furthermore, the first 20 access procedures were observed by the same company representative to ensure that all steps were properly followed. The common femoral artery (CFA) was palpated just below the inguinal ligament. Always under both ultrasound-guidance and fluoroscopy, a puncture site over the upper third of the femoral head was done. The AXERA® 2 system was used according to manufacturer’s protocol [16,17]. In brief, the CFA was first punctured with a micro-access needle and a 0.018 microwire was then advanced. After removing the needle, the AXERA® 2 device was introduced into the CFA. The metal footplate on the device is deployed within the arterial lumen, with ensuing visual verification of blood return through a transparent side arm on the device. Next, the device was pulled back until the footplate was positioned against the vessel wall, ceasing blood flow through the side arm. The AXERA® 2 device was then fired, thus creating a separate, low-angle arteriotomy a few millimeters distal to the initial puncture via a micropuncture needle integrated within the device. Subsequently, the 0.018” microwire was pulled back from the arterial lumen and immediately re-introduced through the back of the device again into the vessel. Next, the footplate is released, and the device is removed. A 5-French sheath is then advanced, later upgraded to a larger sheath as dictated by the intervention performed. Heparin was given immediately after recognition of a lesion to treat and immediately prior to upsizing the sheath. This dose is generally 3000 or 4000 IU depending on patient’s weight. Additional Heparin doses were given depending on case duration, every 45 minutes of procedural time. No activated clotting times (ACTs) were checked during procedural performance. No Heparin reversal was given at completion in all but one patient, and the sheath was removed, applying pressure over a Quickclot (Z-MEDICA, Wallingford, CT) for ten minutes, prolonging the time of pressure depending on visual groin inspection after those ten minutes.
After the procedure, patients are allowed to sit up 30 minutes after completion. This is dictated by the patient’s vital signs and by the groin status after MP. As a routine in uncomplicated patients, they are allowed to stay in bed rest for two hours, and from there they assume a complete sitting up position, for an additional two hours, after which they are allowed to go home.
Within one week after discharge, patients received a postintervention phone call, as a part of the Pima Vascular Quality Control Program, when patients are inquired about their groin status, specifically about bleeding events, visible or palpable hematoma or discomfort. They are also asked about their lower extremities status, and about any possible new events that might have occurred. Our team was notified if the patients visited any Health Care Facility or were admitted in the immediate 30-day post-procedure time, with any vascular or non-vascular complications. This information is recorded in their Electronic Health Records (NextGen Healthcare; NextGen Ambulatory Electronic Health Records version 5.8.2). Patients were generally seen in the Pima Vascular™ clinic about 2 weeks from the procedure, when the PI performed a clinical assessment. If patients reported any access site-related complications, CFA Duplex ultrasound was performed. If no complications were seen, they were scheduled for repeated clinical visits every 3 months for the first year post-procedure, every 6 months for the second year, and yearly thereafter.
Vascular complications were divided into major and minor. Major were defined as: retroperitoneal hemorrhage (confirmed by computed tomography); limb ischemia (loss of peripheral pulse requiring vascular or surgical evaluation); significant groin bleeding (blood loss at the access site resulting in blood transfusion, increased length of stay, or drop in hemoglobin >3 g/dL); nerve injury requiring surgery or permanent in nature; infection requiring an CFA pseudoaneurysm or a groin arteriovenous fistula (confirmed by ultrasonography) or any case requiring access–related surgical intervention. Minor were defined as any groin bleeding not resulting in blood transfusion, not requiring hospital admission or with a verified hemoglobin drop <3 g/dL), groin discomfort without other evidence of vascular problems, or a groin hematoma<6-cm.
Patient demographics, clinical data (including Rutherford classification, following suggested reporting standards), [18] surgical peri-operative data and outcomes were extracted. The primary endpoints of this study were TTH, early sit up time (TTS), TTA, time to discharge (TTD) and major and minor vascular access-related complications. TTH for the ipsilateral access site was defined as elapsed time from sheath removal to first observed cessation of groin bleeding (excluding cutaneous or subcutaneous oozing). TTS was defined as elapsed time from sheath removal and time when the patient sat up at 30-degrees. TTA was defined as elapsed time between sheath removal and time when the patient stands and walks at least 20 feet without re-bleeding. TTD was defined as elapsed time from sheath removal to actual physical discharge from the OBL. Procedure time was defined as elapsed time from the first skin break to final closure (skin-to-skin time).
A comparison between the total group of patients, and the group of those who did not experience any device-related complications was performed to show the expected results with uneventful device use. Furthermore, in order to identify risk factors for successful device use and for the occurrence of access-related complications with its use, univariate analyses were performed. In addition, the complications were analyzed with respect of their time of occurrence, to determine if this was related to the learning curve for this device. With this in mind, the study period was divided in quartiles.
Descriptive statistics were used to report our data. Continuous variables were reported as mean ± SD and categorical variables were expressed in percentage. Data was entered into a computerized spreadsheet [Microsoft Excel 2003, Microsoft corporation, Redmond, WA, USA] and analyzed using SPSS for Mac©, version 19.0 [SPSS, Chicago, IL, USA].
Results
Over the 15-month study period, 129 arterial endovascular interventions were performed. In 18 (13.95%), the intention was to use the AXERA® 2 device but this was not possible. The demographic characteristics of these patients and the reasons for the inability to use the device are summarized on Tables 1 and 2 respectively. Females comprised 55.6% of them, and their average age was 71 years. The most common reason for inability to use was a severely calcified CFA (44.4%) followed by intense groin scarring (38.9%). These cases were then converted to routine access, and an alternative hemostasis method was used, most often MP (n=13, 72.2%) followed by Starclose (n=3, 16.7%) and Proglide (n=2, 11.1%).
Table 1 Demographic characteristics of patients which AXERA could not be used.
| Description | # Patients (Standard Deviation or %) |
|---|---|
| Total number | 18 |
| Age (years) | 71.4 ± 10.1 |
| Body mass Index | 27.9 ± 5.2 |
| Male | 8 (44.4%) |
| Diabetes Mellitus | 8 (44.4%) |
| Hypertension | 16 (88.9%) |
| Hypercholesterolemia | 13 (72.2%) |
| Coronary artery disease | 9 (50%) |
| Current smoker | 3 (16.7%) |
| Former smoker | 8 (44.4%) |
| Clopidogrel | 5 (27.8%) |
| Aspirin | 6 (33.3%) |
| Clopidogrel and Aspirin | 4 (22.2%) |
| Rivaroxaban | 0 (0%) |
| Prasugrel | 1 (5.6%) |
| Any other antiplatelet | 0 (0%) |
| Warfarin | 2 (11.1%) |
| Any antiplatelet or anticoagulant agent | 18 (100%) |
| Chronic kidney disease | 1 (5.6%) |
| On dialysis | 1 (5.6%) |
| Beta blocker | 10 (55.6%) |
| Statin | 12 (66.7%) |
Table 2 Reasons why the AXERA® 2 device could not be used.
| Gender | Age | Date of Procedure | REASON | |
|---|---|---|---|---|
| 1 | Female | 65 | January 12, 2015 | Antegrade/femorofemoral cross over bypass |
| 2 | Male | 75 | January 15, 2015 | Groin scar - Prior femoropopliteal bypass graft |
| 3 | Female | 79 | January 15, 2015 | Antegrade |
| 4 | Female | 74 | January 20, 2015 | Severely calcified common femoral artery |
| 5 | Female | 84 | February 4, 2015 | Severely calcified common femoral artery |
| 6 | Female | 60 | February 17, 2015 | Severely calcified common femoral artery /Proglide |
| 7 | Female | 76 | February 17, 2015 | Severely calcified common femoral artery /Proglide |
| 8 | Female | 58 | February 17, 2015 | Groin scar - prior percutaneous interventions |
| 9 | Male | 87 | April 6, 2015 | Groin scar - prior endovascular aneurysm - Endologix AFXÔ |
| 10 | Male | 58 | April 13, 2015 | Severely calcified common femoral artery /Starclose |
| 11 | Male | 58 | April 27, 2015 | Severely calcified common femoral artery /Starclose |
| 12 | Male | 63 | May 28, 2015 | Groin scar - Prior bypass graft |
| 13 | Female | 60 | May 28, 2015 | Groin scar - Prior iliofemoral endarterectomy |
| 14 | Female | 86 | June 18, 2015 | Severely calcified common femoral artery |
| 15 | Male | 86 | June 29, 2015 | Severely calcified common femoral artery |
| 16 | Male | 64 | November 16, 2015 | Groin scar - Prior bypass graft |
| 17 | Female | 73 | November 16, 2015 | Groin scar - Starclose |
| 18 | Male | 76 | January 20, 2016 | Antegrade access |
Therefore, the AXERA® 2 device could be successfully inserted in 111 (86%) procedures. However, in three of those, only diagnostic procedures were performed and in another three, no lower extremities were intervened (two patients had renal artery revascularization procedures and an additional one underwent CFA access to treat a non-maturing arteriovenous fistula for dialysis). Thus, the remainder 105 interventions (85 patients) represent the cohort studied. The mean age was 73.8 years and 55.2% (n=58) were male. The mean procedural time was 1.5 hours. Their demographic characteristics are summarized on Table 3.
Table 3 Demographic characteristics of patients with AXERA.
| Description | # Patients (SD or %) |
|---|---|
| Total number | 105 |
| Age (years) | 73.8 ± 10.6 |
| Body mass Index | 26.9 ± 5.5 |
| Male | 58 (55.2%) |
| Diabetes Mellitus | 57 (54.3%) |
| Hypertension | 90 (85.7%) |
| Hypercholesterolemia | 72 (68.6%) |
| Coronary artery disease | 58 (55.2%) |
| Current smoker | 27 (25.7%) |
| Former smoker | 26 (24.8%) |
| Clopidogrel | 21 (20%) |
| Aspirin | 18 (17.1%) |
| Clopidogrel and Aspirin | 44(40.4%) |
| Rivaroxaban | 3 (2.9%) |
| Prasugrel | 2 (1.9%) |
| Any other antiplatelet | 1 (1%) |
| Warfarin | 5 (4.8%) |
| Any antiplatelet or anticoagulant agent | 90 (85.7%) |
| Chronic kidney disease | 11(10.5%) |
| On dialysis | 5 (4.8%) |
| Beta blocker | 49 (46.7%) |
| Statin | 55 (52.4%) |
The majority of procedures were performed due to ischemic rest pain (35.2%), followed by life-style limiting claudication (27.6%), minor tissue loss (19%) and major tissue loss (11.4%). This series also included six cases of in-stent re-stenosis (ISR–5.7%) and one of a distal severe anastomotic stenosis of a previously performed femoro-tibial vein bypass (0.9%).
The procedures were performed with 5-, 6- and 7-French sheaths (2.9%, 48.6% and 48.6%, respectively). Intravenous heparin was administered in all interventions (mean dose of 4033 ± 1690 IU) and most patients (85.7%) were taking an antiplatelet or anticoagulant medication at procedural time.
The access site was the right CFA in 52 cases (49.5%), the left in 48 (45.7%) and bilaterally in 5 patients (4.8%). The primary outcomes with unilateral access are shown in Table 4, whereas Table 5 shows our outcomes compared with selected time intervals. The mean radiation time was 287.3 mGy and the mean radiation time was 27.2 minutes.
Table 4 Primary outcomes in patients with unilateral Access.
| Description | All patients | Patients without complications |
|---|---|---|
| Number | 100 | 86 |
| Time to Hemostasis (minutes) | 14.8 ± 6.2 | 13.8 ± 6.2 |
| Time to Ambulation (hours) | 2.4 ± 0.8 | 2.2 ± 0.8 |
| Time to discharge (hours) | 3.8 ± 1.0 | 3.6 ± 1.0 |
Table 5 Outcomes compared with selected time intervals (unilateral access).
| Description | All patients | Patients without complications |
|---|---|---|
| Number of patients | 100 | 86 |
| TTH ≤ 15min | 75 (75%) | 70 (81.4%) |
| TTA ≤ 2.5h | 73 (73%) | 70 (81.4%) |
| TTD ≤ 4h | 73 (73%) | 71 (82.6%) |
Clinical and sonographic follow-up is shown in Table 6. Almost half of our patients (44.8%) had an ultrasound study of the CFA treated with the AXERA® 2 device. This was not a part of our initial protocol since there are no indications to routinely image this vessel unless a symptom or a sign of a problem develops. These studies were mostly done due to the need to image the contra lateral lower extremity prompted by symptoms or signs of ischemia (often our patients did require bilateral extremity interventions) or due to symptoms after arterial puncture. About 85% of patients were physically interviewed and examined by the study PI about 2 weeks after intervention, and about 90% of them were reached over the phone by Pima Vascular Staff within a week after their procedures. On follow-up, four patients died during follow-up (3.8%; seven of the interventions on these patients were included in the final analysis).
Table 6 Cohort follow-up (out of a total of 105 patients).
| TOTAL (n, %) | MEAN (days) | MEDIAN (days) | RANGE (days) | |
|---|---|---|---|---|
| Clinical | 89, 84.8% | 117.43 | 106 | 1-372 |
| Duplex | 47, 44.8% | 113.8 | 105 | 2-372 |
| Phone | 94, 89.5% (from the five patients with complications, three were directly admitted to the hospital and two were admitted after their Pima Vascular discharge. Obviously there was no reason for phone contact on these patients) | |||
A subset of 80.2% (69/86) of patients who did not have any complication after the procedures were allowed to sit up within the first hour after the procedure. Patients were discharged to their homes in 97 interventions, whereas three were discharged to their respective assisted-living homes. Five patients were transferred from Pima Vascular™ or admitted to the hospital setting within the 30-day peri-operative period, with three of them due to access-related complications, one with an acutely ischemic leg due to failure of revascularization unrelated to access (superficial femoral artery stent thrombosis; see below), and a fifth patient who was a very frail gentleman in need of bilateral lower extremity revascularization. He was admitted to the hospital immediately after conclusion of his left lower extremity revascularization, so his right lower extremity could be also revascularized before going back to his home, which is geographically located over two hours away from Pima Vascular™ or the hospital setting. He had no access-related complications after either of both procedures. This adds to 97.1% (102/105) of patients who were discharged to either home, a nursing home or a hospital without the need for additional surgical or interventional procedures to correct access problems, blood transfusions, hemodynamically stable and in good condition upon completion of their interventions.
We had 14 total complications in 13 patients (Table 7). Their demographic characteristics are shown in Table 8. There was an overwhelming female majority (n=11, 78.6%), and their average age was 76 years. All of those developed hematomas > 6-cm in clinical diameter (13.3%). Four patients required hospital admission after their interventions (3.8%). Three of those required immediate hospital transfer whereas the last patient was admitted three days after his discharge from Pima Vascular™, due to acute renal failure, anemia and a femoral artery pseudoaneurysm. Two of those patients required a blood transfusion (1.9%). There were a total of two femoral artery pseudoaneurysms (1.9%), and one of these two patients was the only one in this series to receive intravenous Protamine at completion of his revascularization intervention. They both underwent successful ultrasound-guided thrombin injection, both resolving the pseudoaneurysm at single injection. No infectious or arterial thrombotic complications were seen and no other access complications were identified during a median follow-up of 117 days (1-372 days; Table 6).
Table 7 Access-related complications and their timing (out of a total of 105 patients).
| Age/Gender | Procedure Date | Complication | Hospital Admission* | Pseudoaneurysm | Protamine | Blood Transfusion | |
|---|---|---|---|---|---|---|---|
| 1 | 77/Female | 6/10/15 | Hematoma 60’ after | Yes | No | No | Yes |
| 2 | 92/Fermale | 7/30/15 | Hematoma immediate | No | No | No | No |
| 3 | 77/Female | 8/19/15 | Hematoma post-ambulation | No | No | No | No |
| 4 | 86/Female | 9/1/15 | Hematoma 40’ after | No | No | No | No |
| 5 | 62Female | 9/3/15 | Hematoma, immediate | No | No | No | No |
| 6 | 81/Female | 9/14/15 | Hematoma, immediate | No | No | No | No |
| 7 | 83/Female | 9/17/15 | Hematoma immediate, hypotension | Yes (immediately post procedure) | No | No | No |
| 8 | 67/Female | 10/14/15 | Hematoma immediate, hypotension | Yes (immediately post procedure) | Yes | No | Yes |
| 9 | 69/Female | 12/2/15 | Hematoma 20’ after | No | No | No | No |
| 10 | 69/Male | 12/9/15 | Hematoma 90’ after | No | No | No | No |
| 11 | 59/Female | 12/14/15 | Hematoma 40’ after | No | No | No | No |
| 12 | 83/Male | 1/14/16 | Hematoma, immediate | Yes (3 days post-procedure) | Yes | Yes | Yes |
| 13 | 81/Female | 1/15/16 | Hematoma 15’ after | No | No | No | No |
| 14 | 80/Male | 2/19/16 | Hematoma, immediate | No | No | No | No |
*An additional patient required hospital admission due to acute lower extremity ischemia about a week after OBL revascularization. This patient is not herein included since this was not an access-related complication.
Table 8 Demographic characteristics of patients with complications.
| Description | # Patients (%) |
|---|---|
| Total number | 14 |
| Age (years) | 77.1 ± 10.3 |
| Body mass Index | 26.6 ± 5.2 |
| Male | 4 (28.6) |
| Diabetes Mellitus | 10 (71.4) |
| Hypertension | 12 (85.7) |
| Hypercholesterolemia | 12 (85.7) |
| Coronary artery disease | 9 (64.3) |
| Current smoker | 1 (7.1) |
| Former smoker | 5 (35.7) |
| Clopidogrel | 4 (26.7) |
| Aspirin | 3 (21.4) |
| Clopidogrel and Aspirin | 5 (35.7) |
| Rivaroxaban | 0 (0) |
| Prasugrel | 0 (0) |
| Any other antiplatelet | 1 (7.1) |
| Warfarin | 1 (7.1) |
| Any antiplatelet or anticoagulant agent | 14 (100) |
| Chronic kidney disease | 0 (0) |
| On dialysis | 0 (0) |
| Beta blocker | 8 (57.1) |
| Statin | 5 (35.7) |
None of the analyzed demographic features and peri-operative factors were predictive of successful use of the AXERA® 2 device on univariate analysis (all p>0.05). Specifically, age, BMI, gender, diabetes, hypertension, smoking kidney disease, hypercholesterolemia or preoperative use of antiplatelet medication did not show an independent influence on the ability to successfully use this system. Interestingly however, there was a significant difference among patients in whom the AXERA device was successfully used compared to those in whom this was not possible, in terms of TTH, TTA and TTD (p<0.05) (Table 9). From the same analyzed factors, only the use of >4000IU of intravenous Heparin predicted the occurrence of a postintervention complication (p<0.05). Even though age >80 years did not make statistical significance (p=0.056), it represented the closest demographic factor of all others as a complication predictor (Table 10).
Table 9 Univariate analysis of all demographic features and peri-operative factors vs. inability to use AXERA.
| Description | Patients without AXERA (n=18) | Patients with AXERA device (n=105) | p |
|---|---|---|---|
| Gender (Male) | 8 | 58 | 0.3962 |
| BMI >= 30 | 5 | 24 | 0.8777 |
| Age > 80 years | 4 | 30 | 0.7672 |
| Diabetes Mellitus | 8 | 57 | 0.4396 |
| Hypertension | 16 | 90 | 0.7184 |
| Smoking | 3 | 27 | 0.5969 |
| Hypercholesterolemia | 13 | 72 | 0.7568 |
| Chronic kidney disease | 1 | 11 | 0.091 |
| Use of any antiplatelet | 16 | 85 | 0.4169 |
| TTH >= 15 minutes | 17 | 25 | <0.0001 |
| TTA >2.5 hours | 15 | 27 | <0.0001 |
| TTD > 4 hours | 15 | 27 | <0.0001 |
Table 10 Univariate analysis of all demographic features and peri-operative factors vs. peri-operative complications.
| Description | Patients with complications (n=14) | Patients without complications (n=91) | p |
|---|---|---|---|
| Gender (Male) | 5 | 54 | 0.0972 |
| BMI >= 30 | 3 | 21 | 0.8912 |
| Age > 80 years | 7 | 23 | 0.056 |
| Diabetes Mellitus | 11 | 74 | 0.1994 |
| Hypertension | 13 | 78 | 0.4642 |
| Smoking | 1 | 26 | 0.1678 |
| Hypercholesterolemia | 13 | 60 | 0.1389 |
| Chronic kidney disease | 0 | 11 | 0.3526 |
| Use of any antiplatelet | 12 | 64 | 0.3802 |
| Heparin >4000IU | 7 | 21 | 0.0339 |
| Procedure length >1.5h | 6 | 46 | 0.5920 |
| Rads > 287 mGy | 6 | 25 | 0.2403 |
| Fluoroscopy time > 27 minutes | 4 | 31 | 0.9192 |
| Rutherford >=5 | 7 | 26 | 0.1079 |
When complications were analyzed with respect to the quartile of occurrence, the second and third study quartiles witnessed the largest number of complications (five on each). There was however, no statistical differences when respect to the quartile of occurrence (p>0.05 for all quartiles) (Table 11).
Table 11 Complications analyzed with respect to the quartile of occurrence.
| Quartiles (26 patients each) | Number of complications | % |
|---|---|---|
| First | 1 | 4 |
| Second | 5 | 19 |
| Third | 5 | 19 |
| Fourth | 3 | 11 |
*There is no significant difference between groups (p=0.5721)
Discussion
Vascular closure devices solved some of the problems associated with MP as the sole method of hemostasis after endovascular interventions. The mechanism of action of the AXERA® 2 device falls somewhere in between MC and closure devices. It has been called the self-sealing arteriotomy or a next-generation modified Seldinger technique. It generates a low-angle arteriotomy, thought to enhance tissue-to-tissue contact for a stronger seal after sheath removal. This tissue overlap provides a large area of exposure to tissue factors that promote coagulation and healing, enhancing fibrin generation to serve as the “glue” to seal the narrow lumen tract. Tissue-on-tissue overlap within the arterial wall and blood pressure within the arterial lumen, combined with MP, hastens hemostasis without any foreign body being left behind after sheath removal. One animal study was designed to learn healing microscopic patterns. Patel and colleagues [19] performed AXERA and standard Seldinger access on contra lateral CFAs in sheep, to evaluate potential histologic complications of the longer, lowangle entry as well as healing modes. Serial histologic sections were collected a month after the index procedure. No vascular aneurysms or ectasia, medial dissection, or luminal thrombosis were seen. Healing was expressed by transmural proteoglycan deposition with infiltrating smooth muscle cells accompanied by different levels of angiogenesis, and minimal to mild inflammation. Advanced healing was indicated by the absence of fibrin and near complete endothelial coverage. In the clinical arena, three prior large clinical studies have been published on the use of this device. The RECITAL study [15] enrolled 351 subjects at eight institutions who underwent endovascular interventions with the intention to introduce the AXERA device. Only 5 patients required upsizing the sheath to a size larger than a 6-French. Of the remaining 346 subjects, only 97 (28%) underwent a therapeutic procedure, whereas the rest of patients only had a diagnostic evaluation. No major adverse events were seen, whereas a minor access siterelated complication rate of 4.3% was observed through a 30-day follow-up. A rate of achievement of CFA access using AXERA® 2 followed by sheath placement in the CFA was 97%. TTS was attempted in 73% of subjects and was successfully achieved at 30 minutes in 99.6%. A mean TTH and TTA of 4 minutes and of 1.5 hours respectively compared favorable to literature controls. The SECURE-II trial [20] enrolled 117 subjects with only 36 requiring intervention. A major complication rate of less than 1% was seen and device success was 91.5%. A mean TTH and TTA for diagnostic subjects of 4.6 minutes and of 2.2 hours respectively was again, less than literature controls. One hundred of the patients in this trial underwent a longer follow-up, and were retrospectively assessed for long-term safety. No major complications were observed. Twenty of them required subsequent re-access in the CFA (25 re-access attempts), all successful.
The largest study known to date enrolled 1191 patients worldwide [17]. This cohort also included a large majority of patients who only underwent diagnostic interventions (73%). A mean TTH of 4.9 minutes was shown for all cases, whereas a 2% of overall complication rate was demonstrated. These included one pseudoaneurysm requiring intervention and surgical repair of a damaged arterial branch.
Our study differed from the previous ones in several, significant aspects. We only used the latest generation of the AXERA product, which has undergone several modifications that make its use much more simple and effective. However, our cohort only included “real-life” patients who underwent endovascular therapeutic interventions. These patients all required a larger sheath size (none of them smaller than a 6-French and almost half requiring a 7-French sheath), a bolus of intravenous Heparin and a longer procedural time, all factors that negatively affect hemostatic times. Their baseline characteristics were also adverse, with more than half of them with a diagnosis of diabetes, one-quarter of active smokers and 85.7% of them on some sort of anticoagulant and/or anti-platelet medications. Our cohort features are unique within the arena of prior AXERA® 2 studies.
In our practice, patients generally undergo sonographic assessment prior to the intervention by a very experienced ultrasound technologist, who has been part of the practice for 10 years. Therefore it is quite rare to find patients who undergo solely diagnostic procedures. These patients, in which only a 5-French sheath was used and in which no Heparin was administered, were excluded from this analysis. Despite these antagonist factors, our study results support those of the RECITAL and SECURE-II trials, in that the AXERA® 2 device is safe and effective, even when applied in adverse clinical scenarios. All trials demonstrated a substantial reduction on TTH and TTA compared to historical controls with a very small number of complications.
A recent report however, raised a word of caution in the use of the AXERA device [21]. This report included a very small number of subjects, but an alarmingly high rate of serious complications were reported, including 2 patients in which an arterial dissection caused by the device resulted in CFA occlusions with both patients requiring endarterectomy. This could be theoretically explained by less experienced operators, since only 13 interventions do not speak of a high level of AXERA experience. Other studies with larger subject recruitment have retrospectively reported better outcomes with its use [22]. A single-center study of 94 patients (104 access sites, 81 diagnostic, 23 interventional) undergoing neurovascular procedures using AXERA® 2 device was recently reported. Only 17 procedures (16%) used a 6-F sheath, with the vast majority using a 5-French sheath. About 39% of them did not require intravenous Heparin and only one third of them were on anti-platelet drugs at procedural time. The median TTH was 4 minutes with MP, and outpatients undergoing diagnostic angiography were permitted to ambulate at 1 hour. A 3% rate of complications was seen, including only 1 hematoma and 2 failed procedures. These results correlate better with ours and those reported at the RECITAL and SECURE-II studies.
Our univariate analysis indicate that patients who required larger doses of intraoperative anticoagulation and possibly those older than 80 years of age were likely to experience a complication after AXERA® 2 use. Older patients tend to have more calcification in the femoral arteries which, combined with higher levels of heparinization, makes them more likely to require longer MP to achieve initial hemostasis and hence, higher chances of nonhealing of the puncture site. This will help in patient selection when considering the use of the AXERA® 2 system vs. others for hemostasis.
Study Limitations
A single operator, single institution study implied a very close scrutiny to case details and follow-up. However, a bias towards better outcomes can be feared. Potential patient and operator bias may be inherent in evaluation of some endpoints, for instance in the case of comfort assessment. In the same lines, since these patients reflect the experience of a single operator, these results cannot be extrapolated to every interventionist, since the individual skill and experience level of each operator plays a pivotal role for favorable outcomes. Another limitation is the lack of objective follow-up for most of the accessed CFAs, even though about half of our patients had an ultrasound of the groin treated with the AXERA® 2 device. All of those studies were negative as far as arterial or venous thrombosis, stenosis, pseudoaneurysms, arteriovenous fistulae or any other potential access-related problems. Theoretically, there is a potential higher number of groin complications that were unrecognized on their 1-day phone call follow-up or in their 2-week clinical follow-up. However, we feel that this possibility is quite low since patients were completely asymptomatic. In addition, this study was not randomized and therefore historical controls had to be used in order to confirm safety or efficacy of the AXERA® 2 device. Unfortunately, a randomized controlled trial (the ARISTOCRAT-A trial) to assess the safety and efficacy of this device compared to MP was designed but was ultimately terminated due to financial issues with the manufacturer. Lastly, we are aware that this closure device system is no longer available in the United States market. Nevertheless, this trial serves as validation showing the safety and efficacy of a closure device that leaves “no foreign objects behind” in achieving groin hemostasis.
Conclusions
The AXCELERATE trial showed that the use of the AXERA® 2 system for closure in elective, endovascular lower extremity interventions resulted in a short TTH and TTA, early sit up, and accelerated TTD and TTD without any major complications. In a “real-life” scenario, patients required about 15 minutes of MP immediately after conclusion of the intervention, they ambulated two and a half hours after, and were discharged in less than 4 hours after termination of their procedures. This effectiveness is associated with reported patient comfort, likely related to the ability to sit patients up and to ambulate early.
As operator experience has increased along with advances in peripheral interventional technologies, including adoption of newer closure devices, such as the AXERA® 2 system, the natural evolution has been to perform ever increasing complex procedures in the OBL setting. Caution however, should be exerted in elderly patients who required prolonged, complex interventions that need high doses of Heparin for their performance. Longer MP times might be needed in those cases for adequate hemostasis to be reached.
References
- Anderson PL, Gelijns A, Moskowitz A, Arons R, Gupta L, et al. (2004) Understanding trends in inpatient surgical volume: vascular interventions, 1980-2000. J Vasc Surg 39: 1200-1208.
- Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM (2009) National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 50: 54-60.
- Jain K, Munn J, Rummel MC, Johnston D, Longton C (2014) Office-based endovascular suite is safe for most procedures. J Vasc Surg 59: 186-191.
- Schwartz BG, Burstein S, Economides C, Kloner RA, Shavelle DM, et al. (2010) Review of vascular closure devices. J Invasive Cardiol 22: 599-607.
- Simon A, Bumgarner B, Clark K, Israel S (1998) Manual versus mechanical compression for femoral artery hemostasis after cardiac catheterization. Am J Crit Care 7: 308-313.
- Ward SR, Casale P, Raymond R, Kussmaul WG, Simpfendorfer C (1998) Efficacy and safety of a hemostatic puncture closure device with early ambulation after coronary angiography. Am J Cardiol 81: 569-572.
- Schickel SI, Adkisson P, Miracle V, Cronin SN (1999) Achieving femoral artery hemostasis after cardiac catheterization: a comparison of methods. Am J Crit Care 8: 406-409.
- Bavry AA, Raymond RE, Bhatt DL, Chambers CE, DeNardo AJ, et al. (2008) Efficacy of a novel procedure sheath and closure device during diagnostic catheterization: the multicenter randomized clinical trial of the FISH device. J Invasive Cardiology 20: 152-156.
- Hermiller J, Simonton C, Hinohara T, Lee D, Cannon L, et al. Clinical experience with a circumferential clip-based vascular closure device in diagnostic catheterization. J Invasive Cardiology 17: 504-510.
- Dauerman HL, Applegate RJ, Cohen DJ (2007) Vascular closure devices: the second decade. J Am Coll Cardiol 50: 1617-1626.
- Doyle BJ, Godfrey MJ, Lennon RJ, Ryan JL, Bresnahan JF, et al. (2007) Initial experience with the Cardiva Boomerang vascular closure device in diagnostic catheterization. Catheter Cardiovasc Interv 69: 203-238.
- Madigan JB, Ratnam LA, Belli AM (2007) Arterial closure devices. A review. J Cardiovasc Surg 48: 607-624.
- Derham C, Davies JF, Shahbazi R, Homer-Vanniasinkam S (2007) Iatrogenic limb ischemia caused by angiography closure devices. Vasc Endovascular Surg 40: 492-494.
- Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW (2001) Complications of femoral artery closure devices. Catheter Cardiovasc Interv 52: 3-7.
- Bangalore S, Arora N, Resnic FS (2009) Vascular closure device failure: frequency and implications: a propensity-matched analysis. Circ Cardiovasc Interv 2: 549-556.
- Turi ZG, Wortham DC, Sampognaro GC, Kresock FD, Held JS, et al. (2013) Use of a novel access technology for femoral artery catheterization: results of the RECITAL trial. J Invasive Cardiol 25: 13-18.
- Thilo T, Joachim S (2010) TCT-335: Novel arterial puncture and closure system: First experience in 1000 patients worldwide. J Am Coll Cardiol 56: B78.
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, et al. (1997) Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 26: 517-538.
- Patel R, Virmani R, Kolodgie FD, Cogan D (2013) Vascular healing after AXERA Access and Closure: a 30 day histopathological assessment in the ovine model. J Am Coll Cardiol 62: B242.
- http://www.arstasis.com/wp-content/uploads/pdfs/LBL-04003-F.pdf
- Grandhi R, Zwagerman NT, Zhang X, Chen SH, Jadhav AP, et al. (2015) Initial experience with the AXERA 2 Femoral Access System in neurovascular procedures. Interv Neuroradiol 21: 412-417.
- Fortes MC, Jindal G, Polifka AJ, Aldrich EF, Simard JM, et al. (2013) Low-angle vascular access for neurovascular procedures using the Arstasis AXERA access device. J Vasc Interv Radiol 24: 693-697.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences