Arteriovenous Malformation: Concepts on Physiopathology and Treatment
Neto CASF and Durans M
Published Date: 2019-04-30DOI10.21767/2573-4482.19.04.6
Department of Vascular and Endovascular Surgery, Presidente Dutra Hospital, São Luís, Brazil
- *Corresponding Author:
- Carlos Alberto da-Silva Frias Neto
Department of Vascular and Endovascular Surgery
Presidente Dutra Hospital, São Luís-Maranhão, Brazil
Email: friasneto@gmail.com
Received date: January 14, 2019; Accepted date: February 19, 2019; Published date: February 26, 2019
Citation: Neto CASF, Durans M. Arteriovenous Malformation: Concepts on Physiopathology and Treatment. J Vasc Endovasc Therapy. 2019, Vol.4 No.1:6.
Copyright: © 2019 Neto CASF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Arteriovenous malformations represent a complex type of vascular anomalies, caused by an abnormal angiogenesis. Genetic disorders are already catalogued for specific situations. These lesions can be classified according to its angio-architecture and clinical presentation. Patients with progressive symptoms according to Schobinger classification require treatment. Surgical treatment is indicated when total resection of the nidus is viable, which is not often. Embolization became the first-choice therapy, as it offers a way of treatment with low morbidity and acceptable results. Many devices are used to achieve nidus occlusion, but liquid embolic devices are the agents of choice. Total occlusion of the AVM is important to avoid stimulate more angiogenesis and lesion recurrence.
Keywords
Arteriovenous; Malformation; Nidus
Introduction
Through access to PubMed and other relevant works of the literature, a search for articles on the mechanisms of arteriovenous malformations (AVMs) was conducted, emphasizing the information regarding peripheral AVMs. The terms "arteriovenous malformation" and "nidus" were used to collect relevant publications.
Arteriovenous malformations (AVMs) are anomalous communications between arteries and veins that result from angiogenesis disorders. They are characterized by the presence of abnormal communications between the arterial and venous systems, without the interposition of the capillary network. These shunts, in most cases, are multiple and are configured as a mass of intrinsically related vessels, a true conglomerate of vessels known as vascular nidus [1].
The pathogenesis of AVMs is an abnormal angiogenesis during the embryonic period associated with an action of vascular growth factors. Elevated levels of endothelial growth factor (VEGF) have been demonstrated in nidus and adjacent cells in cerebral AVMs [2]. Elevated levels of VEGF were also demonstrated in the plasma of patients with AVM [3].
Literature Review
Numerous causal genes have been discovered and associated with numerous vascular anomalies. According to ISSVA last classification, from May 2018, there are 41 genes already catalogued associated with peripheral vascular anomalies. Referring specific to AVMs, the subsequent genes are already catalogued: MAP2K1 for sporadic MAVs, RASA1 and EPHB4 for composite capillary-arteriovenous malformation and HHT1 ENG, HHT2 ACVRL 1, HHT3, JPHT SMAD4 for Hereditary hemorrhagic telangiectasia (HHT) [4].
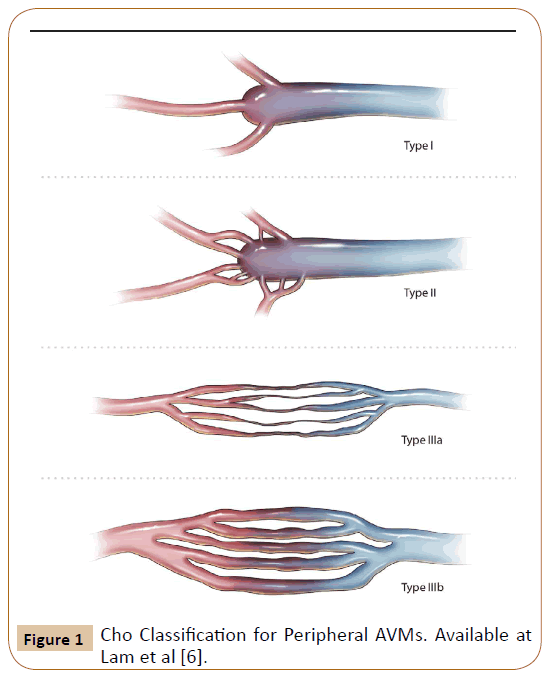
Peripheral AVMs are classified by the Cho scale [5], depending on the angioarchitecture formed between the nourishing arteries, the nidus and the drainage veins. This architecture in angiography is taken as the basis for therapeutic and prognostic decisions. Cho described 4 distinct architectures (Figure 1) and demonstrated that AVMs with multiple arterial branches that flow into a single vein, types 1 and 2, have a better response to treatment. AVMs with multiple inflows and outflows, types 3a and 3b, have the worst response [6].
Figure 1: Cho Classification for Peripheral AVMs. Available at Lam et al [6].
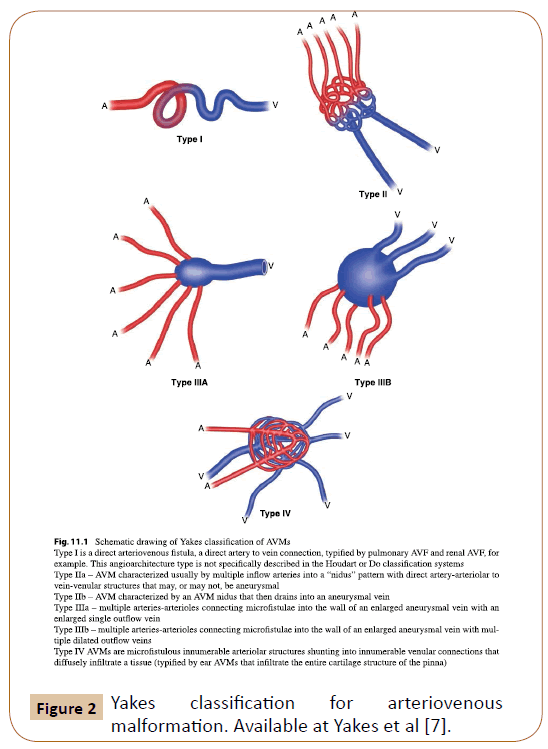
Yakes developed a new classification (Figure 2) of the angioarchitecture of these lesions. The AVM Classification System is used to determine endovascular approaches and the embolic agents that will be successful to ablate these AVMs [7]. The classification of Yakes included lesions that by ISSVA classification are grouped as arteriovenous fistulas (AVFs) and not as AVMs (according to ISSVA Classification from 2018 [4]).
Figure 2: Yakes classification for arteriovenous malformation. Available at Yakes et al [7].
Frey S. et al found evidence for the existence of a hyperdynamic, capillary-venulous malformation (CV-AVM), what would it be different from any AVM angioarchitecture described and classified so far. They developed a computational model to study the effects of micro vascular anomalies on local hemodynamics, as well as their impact on angiographic contrast propagation. Their results shows that the hypothesized CV-AVM morphology of enlarged, fistulous paths on the venous half of capillaries and/ or dilated draining venules, with similar characteristics of type IV AVMs together with features that are commonly associated with venous malformations. DSA recordings show diffuse, “cloudy” regions of CA accumulation similar to type IV AVMs, but venous drainage is more delayed and dispersed [8].
Injuries present from birth, AVMs grow along with the patient and may also be stimulated by local trauma, hormonal changes and infections [6]. AVMs can develop anywhere in the body and present diverse clinical pictures, from a small asymptomatic lesion to an injury with severe impairment of vital functions.
In general, these lesions present as pulsatile masses with increased temperature, and may also be associated with fremitus. There may be bone and soft tissue growth surrounding the lesion. In advanced stages, trophic lesions may develop, due to the theft of flow caused by AVM and due to venous hypertension [9].
According to the clinical presentation of the pathology, Schobinger [10] classified AVMs as follows at Table 1.
| Stage | Clinical Findings |
|---|---|
| I (Quiescence) | Warm, pink-blue, shunting on doppler |
| II (Expansion) | Enlargement, pulsation, thrill, bruit, tortuous veins |
| III (Destruction) | Dystrophic skin changes, ulceration, bleeding, pain |
| IV (Decompensation) | Cardiac failure |
Table 1: Schobinger staging of AVM [10].
Peripheral AVMs are diagnosed primarily by vascular Doppler ultrasound and MRI. Doppler flow pattern analysis is extremely important to determine the hemodynamic characteristics of AVMs with high systolic and diastolic (biphasic) velocities, indicating low resistance flow [1]. In MRIs, AVMs appear as a tangle of arteries and dilated veins connected by shunts (nidus). These vessel networks demonstrate flow void in T1 and T2 and appear as a hyperintense image in T2, indicating high flow lesions. Unlike other vascular malformations, AVMs do not show enhancement of adjacent soft tissues at T2, except when edema is present. T1 images are also useful for defining AVM morphology, demonstrating vessel enhancement after contrast and early venous filling. MRI is an important exam in preoperative evaluation and postoperative control [11]. Computed tomography (CT) can be performed in patients with pulmonary AVFs or in AVMs with bone involvement. Conventional arteriography is usually performed immediately before interventional treatment [12]. The classic appearance of AVMs in angiography demonstrates multiple dilated nourishing arteries, nidus and early opacification of dilated drainage veins [11].
Invasive therapy is indicated in patients with progressive symptoms according to the Schobinger classification. Surgical treatment is indicated for peripheral AVMs when total nidus resection is possible, which is a rare condition. Most often, the patient has a lesion with high hemorrhage risk to operate or has multiple lesions, so that the surgical treatment is generally of high risk or it promotes partial and suboptimal resection of the lesions. Partial resections may cause a positive clinical response initially, but over time the patient eventually develops recurrence of symptom and may even present worsening of the condition. This is why embolization became the first-choice therapy, as it offers a way of treatment with low morbidity and acceptable results.
The goal of endovascular embolotherapy is to occlude the nidus or fistula completely, redirecting vascular flow to normal vessels. Commonly used agents are absolute alcohol, N-butyl cyanoacrylate (NBCA or cola), ethylene-vinyl-alcohol-copolymer (EVOH, Onyx, Squid). In addition, springs or plugs can be used as adjuvants, since these devices occlude only afferent or efferent vessels and never the nidus properly. The interventionalist must be aware of the different delivery mechanisms and material properties. Many authors use a combination of the following embolic agents for the endovascular treatment of complex fastflow malformations [12].
Lam et al., [6] recommends treatment based on the angioarchitecture of the lesions of Cho's classification. In AVMs with single vein outflow, types I and II, retrograde venous access catheter or by direct puncture are the methods of choice. The flow is initially reduced by manual compression of the drainage vein or by pressure gauging cuff. When such flow control methods are not possible, use of intravascular balloon may be helpful. In large aneurysmal drainage veins, springs and glue have been successfully used to occlude the outflow. In small AVMs of this type, the use of glue can be used successfully. In most cases, after occlusion of the outflow, retrograde filling of the nidus can be obtained with absolute alcohol or Onyx.
For AVMs with multiple supply arteries and draining veins, types 3a and 3b a trans arterial or direct puncture is recommended. Direct puncture may be necessary in situations where the tortuosity of the nourishing arteries or any other factors hinders the intranodal position of the microcatheter. Decreased or interrupted flow is considered essential to ensure adequate contact of the sclerosant in the nidus. Manual compression of venous drainage or use of arterial cuff may be necessary. A combined approach of trans arterial injection of glue followed by direct puncture of AVM with intranidal direct injection of sclerosant.
Yakes [13] also recommends possible therapeutic lines based on his classification. Yakes follows different behaviors of other services, using as main therapeutic agent the ethanol (Table 2).
| Type of lesion | Therapeutic possibilities |
|---|---|
| Yakes Type I | It can be permanently occluded with mechanical devices like springs and Amplatzer plugs. Ethanol alone can be used in small diameter FAVs. |
| Yakes Type II | It can be treated trans arterially with undiluted ethanol. In some cases, balloons, tourniquets or blood pressure cuffs may be used to slow flow in the nidus and facilitate the use of ethanol. Direct puncture of the lesion is also viable. |
| Yakes Type IIIa | It can be treated trans arterially with undiluted ethanol similarly to the treatment performed on Yakes Type IIa AVM lesions. They may also be permanently occluded with venous aneurysm embolization with springs, with or without embolization with ethanol. This can be obtained by direct puncture of the lesion (percutaneous) or venous retrograde catheterization until venous aneurysm. |
| Yakes Type IIIb | It can be permanently occluded by trans arterial access as in the Yakes Type II AVMs lesions. Embolization of venous aneurysm and spinal drainage veins may be performed. |
| Yakes Type IV | It can be permanently occluded with super selective arterial catheterization and embolization with a mixture of 50% of non-ionic contrast and 50% of ethanol, thus treating the micro-fistulas and sparing the capillaries (vessels of greater resistance). Direct puncture (percutaneous) with needles 23 gauge in the lesion and ethanol injection is also possible and curative. |
Table 2: Yakes therapeutic recommendations for AVMs [13].
Although liquid embolizations shows good results in many situations, in some cases it does not works well, and in some cases the nidus can even develop again. Incomplete embolization of an AVM results in local and regional hypoxia in the obliterate portion of the nidus. It has been demonstrated that this stress caused by hypoxemia can initiate a cascade of events leading to increased VEGF expression and a stimulus to post-embolization angiogenesis. The cascade of inflammatory events that occur in AVM after embolization may be another mechanism for angiogenesis. After embolization, a variety of inflammatory changes occur in the AVM tissue. Embolization induces an inflammatory reaction in the wall of the AVM vessels, which can generate a high potential of angiogenesis in the AVM tissues. Another factor involved is the change in hemodynamic stress forces in the afferent or efferent vessels of AVM after embolization, which may also induce a process of angiogenesis [14].
Based on the concept that AVMs are dynamic entities with constant remodeling induced by the process of angiogenesis, and because the regeneration of these structures are also stimulated by angiogenesis, the use of anti-angiogenic drugs such as VEGF blockers has been discussed as a therapy for the treatment of this disease [15,16].
Regardless of the approach used for treatment, it is important that the physician understands well the mechanisms that govern the functioning of these lesions. Inappropriate treatment can lead to worsening of the lesions, and, in certain cases, make impossible new therapeutic options.
Conclusion
Vascular malformations and its nidus are not only a disarranged communication between arteries and veins but also a physiopathological structure able to stimulate angiogenesis, maintaining and expanding its structure. Besides the development of new devices and new techniques for its treatment, it persists being an anomaly of difficult resolution.
References
- Carnevale FC (2017) Treatment of interventional radiology and endovascular surgery. Thieme Revinter Publications LTDA. Koizumi T, Shiraishi T, Hagihara N, Tabuchi K, Hayashi T, et al. (2002) Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurg 50: 117-126.
- Sandalcioglu IE, Wende D, Eggert A, Müller D, Roggenbuck U, et al. (2006) Vascular endothelial growth factor plasma levels are significantly elevated in patients with cerebral arteriovenous malformations. Cerebrovasc Dis 21: 154-158.
- https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf
- Cho SK, Do YS, Shin SW, Kim DI, Kim YW, et al. (2006) Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. J Endovasc Ther 13: 527-538.
- Lam K, Pillai A, Reddick M (2017) Peripheral arteriovenous malformations: Classification and endovascular treatment. Appl Radiol 46: 15-21.
- Yakes WF, Vogelzang RL, Ivancev K, Yakes AM (2017) New arteriographic classification of AVM based on the yakes classification system. In Congenital Vascular Malformations 63-69.
- Frey S, Cantieni T, Vuillemin N, Haine A, Kammer R, et al. (2018) Angioarchitecture and hemodynamics of microvascular arterio-venous malformations. PloS one 13: e0203368.
- Giménez M (2011) Manual de técnicas intervencionistas guiadas por imágenes. Ediciones J.
- Kohout MP, Hansen M, Pribaz JJ, Mulliken JB (1998) Arteriovenous malformations of the head and neck: natural history and management. Plast Reconstr Surg 102:643-654.
- Mulligan PR, Prajapati HJ, Martin LG, Patel TH (2014) Vascular anomalies: classification, imaging characteristics and implications for interventional radiology treatment approaches. Br J Radiol 87: 20130392.
- Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA (2018) Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular Malformations. InRöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 190: 927-937.
- Yakes WF, Yakes AM, Vogelzang RL, Ivancev K (2017) Endovascular Treatment of Vascular Malformation: An Overview. In Congenital Vascular Malformations: 197-209.
- Khaw AV, Mohr JP, Sciacca RR, Schumacher HC, Hartmann A, et al. (2004) Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke 35: 660-663.
- Vernimmen FJAI (2014) Vascular endothelial growth factor blockade: A potential new therapy in the management of cerebral arteriovenous malformations. J Med Hypotheses Ideas 8: 57-61.
- Walker EJ, Su H, Shen F, Degos V, Amend G, et al. (2012) Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke 43:1925-1930.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences