Coexisting Aortoenteric and Aortovesical Fistulae after Endovascular Aortic Repair of Abdominal Aortic Aneurysm: A Case Report
Heng-hui Yin, Yang Zhao, Mian Wang, Shen Ming Wang and Guang Qi Chang
DOI10.21767/2573-4482.100025
Division of Vascular Surgery, The First Affiliated Hospital, SUN Yat-sen University, Guangzhou, China
- *Corresponding Author:
- Chang Guangqi
Division of Vascular Surgery
The First Affiliated Hospital, SUN Yat-sen University
Guangzhou, China.
Tel: 008615813383001
E-mail: changvascular@126.com
Received Date: September 15, 2016; Accepted Date: October 04, 2016; Published Date: October 11, 2016
Citation: Yin H, Zhao Y, Wang M, et al. Coexisting Aortoenteric and Aortovesical Fistulae after Endovascular Aortic Repair of Abdominal Aortic Aneurysm: A Case Report. J Vasc Endovasc Surg. 2016, 1:4. doi: 10.21767/2573-4482.100025
Abstract
Introduction: Aortoenteric or aortovesical fistulae often occurred primarily with abdominal aorta aneurysm (AAA). Literature retrieval indicated that this might be the first report of such complication secondary to Endovascular aorta repair (EVAR).
Case presentation: A 71 year-old man who had accepted EVAR for AAA 2 years ago suffered from persistent fever, abdominal pain, hematuria and intermittent bloody stools. Cystoscopy revealed a fistula located at the bladder dome with persistent low-grade blood flow. Computed tomographic angiography (CTA) revealed a large AAA with slight type I B endoleak located within the distal end of left external iliac artery stent. Contrast was detected within the bowel and bladder. After strict antibiotic preparation, the patient received axillo-bifemoral arterial bypass, graft excision, bladder repair, and partial resection of the ileum. The patient was successfully treated and no severe complications occurred during the 2 years follow-up.
Conclusion: Co-existing aortoenteric and aortovesical fistulae are extremely rare complication following EVAR. Surgical treatment is vital to avoid fatal results.
Keywords
Aortoenteric fistula; aortovesical fistula; Abdominal aortic aneurysm; Endovascular aortic repair
Introduction
Traditionally, secondary aortoenteric fistula (AEF) and aortovesical fistula (AVF) are rare complications of abdominal aortic aneurysm (AAA) repair which can lead to life-threatening gastrointestinal bleeding or hematuria [1]. Few reports exist in the literature regarding AEF or AVF formation following endovascular aortic repair (EVAR). The main presentations of AEF and AVF are hemorrhage and infection. We presented a case of a 71 year-old man diagnosed with secondary coexisting aorto-enteric and vesical fistulae after EVAR of an AAA which was successfully treated with axillo-bifemoral arterial bypass, graft excision, repair of the bladder, and partial iliac resection. Literature retrieval indicated that no such cases had ever been reported before.
Case Presentation
The patient consented to publishing this report
A 71 year-old man was admitted to our center complaining of low-grade abdominal pain for approximately 1 year. His past medical history included EVAR for AAA (diameter of about 65 mm) 2 years prior to his presentation without any signs of AEF or AVF, and percutaneous nephrolithotomy 12 years ago at another center. He also had a history of hypertension for over 10 years.
Clinical palpation revealed a pulsatile abdominal mass and interval computed tomographic angiogram (CTA) showed a distal type I B endoleak from left iliac extension limbs and type II endoleak from left internal iliac artery. The diameter of the AAA sac was approximately 75 mm. He underwent left internal iliac artery ligation extraperitoneally and left external iliac artery endografting with a 12×80 mm covered stent (Fluency, Bard Co.) with successful obliteration of the type II endoleak and significant decrease in the type I B endoleak.
One month later, this patient again presented to our center complaining of 3 weeks of intermittent bloody stools, 2 weeks of hematuria, and 2 weeks of low-grade persistent abdominal pain and fever. Vital signs included a blood pressure of 105/65 mmHg, a pulse rate of 90 beats/min, and a body temperature of 38.2°C. Clinical palpation revealed abdominal tenderness and an abdominal mass without pulsation. Laboratory investigation indicated a hemoglobin of 72.0 g/L (normal, 110.0-130.0 g/L), red blood cell specific volume (HCT) of 0.22 (normal, 0.42- 0.49), C reactive protein of 73 mg/L (normal, 0-10 mg/L), white blood count of18840 cell/mm3 (normal, 4000-10000 cell/mm3) and neutrophil percentage of 81.1% (normal, 46.0-75.0%). The prothrombin time (PT) and activated partial thromboplastin time (APTT) were 14.7s (normal, 11-14s) and 38.8s (normal, 25-35s), respectively, and 4+ positive blood was detected in the urine.
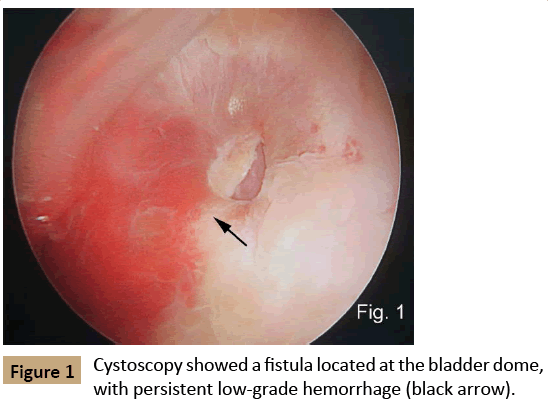
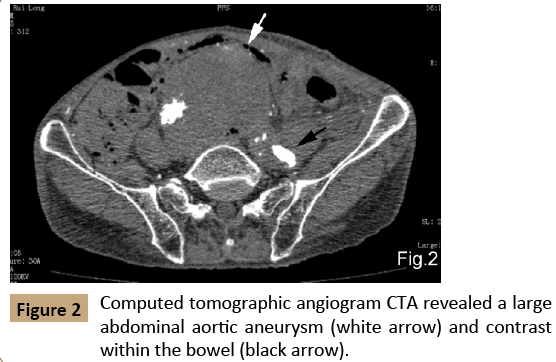
Cystoscopy showed a fistula located at the bladder dome with persistent low-grade blood flow (Figure 1). Gastroscopy and colonoscopy were also performed but the fistula site could not be identified. CTA revealed a large AAA (dilated to 80 mm in diameter) within the distal aorta, with type I B endoleak located within the distal end of left external iliac artery stent. Contrast was detected within the bowel and bladder but the leakage point could not be identified (Figure 2).
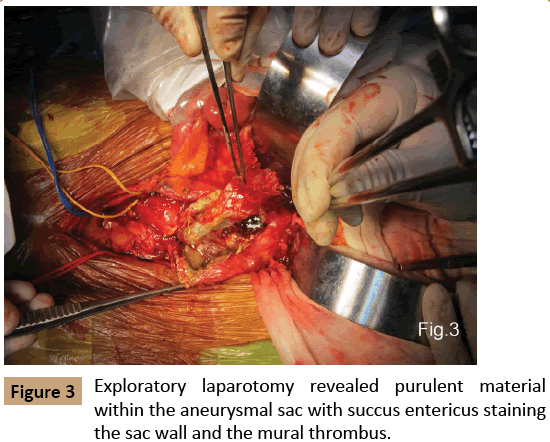
Surgery was performed after five days of antibiotics (imipenem, cefoperazone) were administered to treat the infection. The patient initially underwent a right axillo-bifemoral artery bypass. Following abdominal exploratory revealed two fistulae between the large AAA and the lower section of ileum and dome of the bladder. There was purulent material within the aneurysmal sac with succus entericus staining the sac wall and within the mural thrombus (Figure 3). Low blood flow from the seal zone of left external iliac artery stent was identified, confirming a type I B endoleak. The graft was removed without difficulty, and the proximal as well as distal aortic stumps were ligated in two layers. After local irrigation with antibiotic, primary partial resection of the fistulated ileum and repair of the fistulated bladder were successfully completed. Culture of the fistulae revealed Enterococcus Faecium.
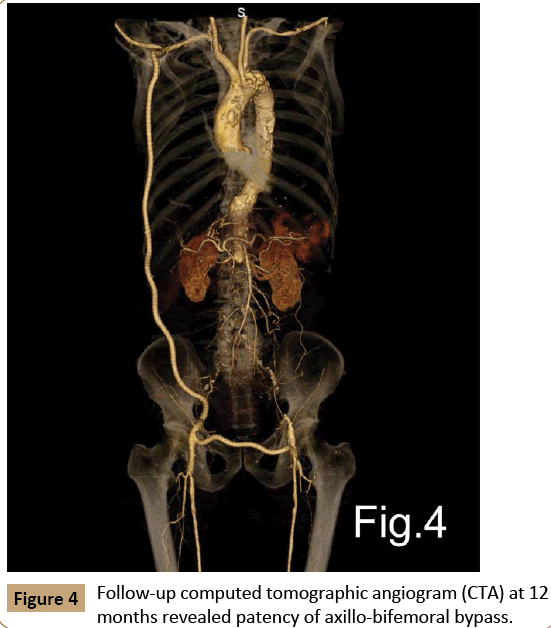
The patient was transferred to our surgical intensive care unit (SICU) for further care. During his time in SICU, he was treated with 7 days of broad-spectrum antibiotics (imipenem, vancomycin, fluconazole), and followed by oral antibiotics (cefixime) for 3 weeks. He was transferred from SICU to the regular floor on postoperative day 8 and discharged on day 21 on life-long oral anti-platelet therapy (aspirin, clopidogrel). The patient recovered well during 2 years follow-up without any signs of graft or abdominal infection. Follow-up CTA at 3, 6 and 12 months, respectively, after discharge revealed patency of both bypasses (Figure 4).
Discussion
Post-EVAR AEF remains an extremely rare condition with only limited reports in the literature [2-9]. Post-EVAR AVF is an even rarer phenomenon with even fewer reports in the literature. Co-existing AEF and AVF secondary to EVAR had never been reported. The etiology of fistulae formation after EVAR is usually abrasion and erosion of adjacent iliac loop, ureter, or bladder caused by transluminal aortic coils [7], type I or II endoleaks with persistent AAA sac expansion [3,5,10], and graft kinking or erosion [4],which theoretically share much in common with AVF formation. The specific risk factors which contribute to primary AVF formation are pelvic surgery, ureteral stenting, and previous radiation therapy [11], and these factors also increase the risk of post-EVAR AVF formation.
The classic manifestations of both AEF and AVF are brisk gastrointestinal bleeding, hematuria, abdominal pain, and persistent low-grade fever. Most existing AEFs present with rapid hematemesis and significant hemodynamic compromise and shock [4,5,7]. Many cases require blood transfusion and AVF cases still require catheterization and bladder washout. Although our patient presented with 3 weeks of intermittent gastrointestinal hemorrhage related to the AEF and 2 weeks of hematuria related to AVF after EVAR, he remained stable despite the anemia. We believe the persistent low-flow distal type I endoleak caused progressive enlargement of the AAA sac and erosion into the lower section of ileum and dome of the bladder. Because the endoleak came from the distal end of stent located within the left external iliac artery, the pressure within the aneurysm sac was very low. Thus, the patient’s hemorrhage manifested as low-pressure and intermittent self-limited gastrointestinal hemorrhage and hematuria as opposed to the systematic aortic pressure present in most classic AEF or AVF patients.
Various procedures have been used to treat AEF and AVF such as simple repair of aortic wall with local antibiotic irrigation [12], in situ aortic reconstruction using femoral-popliteal vein graft or cryopreserved graft, axillobifemoral artery bypass, hybrid technique [13], or complete endovascular repair [14,15]. Opening surgical procedure of simple repair of aortic wall, in situ aortic reconstruction, and axillobifemoral artery bypass have already been well described. Nomoto et al. reported a primary ureteroaortic fistula treated with primary repair of the aortic wall and nephroureterectomy [12]. Sorelius et al. successfully treated an acute secondary AEF, due to remodeling of a stent graft, using a hybrid technique with endovascular repair combined with open enterorrhaphy, omental flap coverage of the stent graft, and local antibiotic irrigation [13]. The patient was free from infectious or hemorrhagic events during four years follow-up. Bas et al. successfully performed a complete EVAR in a case of primary AEF [14]. However, there were still air bubbles within the aneurysm sac according to the follow-up CTA, and this patient died of myocardial infarction with only nine months follow-up. Therefore, mid- to long-term prognosis of endovascular repair of AEF still remained unclear.
After the electronic literature review, scant evidence can be found in the published literature discussing the treatment of post-EVAR AEF or AVF. In our opinion, considering the possible contaminated or infected condition of local tissue (including aneurysm sac and stent graft), there will be extremely high risk of inner-stent infection after endovascular repair of AEF or AVF. Therefore, our suggestion for treatment options of such complication is as following:
• Once the secondary AEF or AVF after EVAR of AAA is confirmed, opening surgery should be considered as first choice dealing with such lesion.
• Endovascular repair should not be considered as a choice dealing with any type of endoleak for AAA patient with secondary AEF or AVF, except for rapid hemorrhage control in hemodynamically unstable patient.
• If the hemodynamical condition is stable after emergency endovascular repair, rigorous CTA follow-up should be performed and opening surgical re-intervention should be prepared at any time.
In our case, AEF and AVF were coexistent and purulence could be found within the aneurysmal sac, indicating a severe infection. In addition, the thrombus within the aneurysm sac was a good medium for pathogens from the intestinal tract and urinary tract. Therefore, in situ reconstruction was not an option in our patient. Endovascular repair of a secondary AEF or AVF has emerged as a new method of rapid hemorrhage control in hemodynamically unstable patients. But we believe it should be regarded as a temporary procedure, for the fistulae still exist whether the endoleak has been treated or not. Pathogens from both intestinal and urinary tracts can still migrate into the aneurysmal sac and the risk of mid- to long-term infection in such a patient is still high. Thus, we selected axillobifemoral artery bypass and local antibiotic irrigation as our surgical regimen.
The mid to long-term patency of the bypass grafts and infection were the two main concerns in this patient. Life-long oral antiplatelet therapy was required and the bypass grafts remained patent during follow-up without any sign of abdominal or systemic infection.
Conclusion
Coexisting AEF and AVF after EVAR of AAA is an extremely rare condition. Progressive aneurysmal sac enlargement resulting from endoleak or endotension may be the main reason. Extraanatomic bypass, graft excision and local debridement are of key importance to avoid fatal outcome and further infection. If the patient was hemodynamically unstable, endovascular stent grafting would have been an alternative, but temporary method to control the rapid hemorrhage.
References
- Bergqvist D, Bjorck M (2009) Secondary arterioenteric fistulation--a systematic literature analysis. Eur J Vasc Endovasc Surg 37: 31-42.
- Makar R, Reid J, Pherwani AD, Johnston LC, Hannon RJ, et al. (2000) Aorto-enteric fistula following endovascular repair of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 20: 588-590.
- Abou-Zamzam AJ, Bianchi C, Mazraany W, Teruya TH, Hopewell J, et al. (2003) Aortoenteric fistula development following endovascular abdominal aortic aneurysm repair: a case report. Ann Vasc Surg 17: 119-122.
- Norgren L, Jernby B, Engellau L (1998) Aortoenteric fistula caused by a ruptured stent-graft: a case report. J Endovasc Surg 5: 269-272.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D (2008) Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 15: 441-448.
- Hausegger KA, Tiesenhausen K, Karaic R, Tauss J, Koch G (1999) Aortoduodenal fistula: a late complication of intraluminal exclusion of an infrarenal aortic aneurysm. J Vasc Interv Radiol 10: 747-750.
- Elkouri S, Blair JF, Therasse E, Oliva VL, Bruneau L, et al. (2003) Aortoduodenal fistula occurring after type II endoleak treatment with coil embolization of the aortic sac. J Vasc Surg 37: 461-464.
- Janne DB, Soula P, Otal P, Cahill M, Joffre F, et al. (2000) Aortoduodenal fistula after endovascular stent-graft of an abdominal aortic aneurysm. J Vasc Surg 31: 190-195.
- Ruby BJ, Cogbill TH (2007) Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 45: 834-836.
- McPhee JT, Soybel DI, Oram RK, Belkin M (2011) Primary aortoenteric fistula following endovascular aortic repair due to type II endoleak. J Vasc Surg 54: 1164-1166.
- Batter SJ, McGovern FJ, Cambria RP (1996) Ureteroarterial fistula: case report and review of the literature. Urology 48: 481-489.
- Nomoto T, Tanaka Y, Yamamoto K (2009) Uretero-aortic fistula: a case report. Tokai J Exp Clin Med 34: 12-14.
- Sorelius K, Sundbom M, Mani K, Wanhainen A (2014) Hybrid treatment of a post-EVAR aortoenteric fistula. Vascular 22: 385-389.
- Bas A, Simsek O, Kandemirli SG, Rafiee B, Gulsen F, et al. (2015) Evolution of computed tomography findings in secondary aortoenteric fistula. Iran J Radiol 12: e22759.
- Williams CA, Zatina MA (2014) Endovascular stent graft deployment as the initial intervention for a bleeding aortoenteric fistula. Am Surg 80: e207-e209.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences