Predicting Stroke Risk in Patients with Carotid Artery Stenosis Using Contrast Enhanced Carotid Duplex Ultrasound to Quantify Plaque Vasa-Vasorum Volume: Results of a Pilot Study
Mahmoud B Malas, Isibor J Arhuidese, Besma Nejim, Umair Qazi, Tammam Obeid, Maya Salameh, Bruce Perler and Alexander Nodel
DOI10.21767/2573-4482.100048
Mahmoud B Malas*, Isibor J Arhuidese, Besma Nejim, Umair Qazi, Tammam Obeid, Maya Salameh, Bruce Perler and Alexander Nodel
Department of Surgery, The Johns Hopkins Medical Institution, Baltimore, MD, USA
- *Corresponding Author:
- Mahmoud Malas
Department of Surgery
Division of Vascular Surgery
Johns Hopkins Medical Institution
4940 Eastern Ave, A 547 Baltimore
MD 21401, USA
Tel: (410) 550-5524
Fax: (410) 550-1274
E-mail: bmalas1@jhmi.edu
Received Date: February 09, 2017; Accepted Date: May 18, 2017; Published Date: May 25, 2017
Citation: Malas MB, Arhuidese IJ, Nejim B, et al. Predicting Stroke Risk in Patients with Carotid Artery Stenosis Using Contrast Enhanced Carotid Duplex Ultrasound to Quantify Plaque Vasa-Vasorum Volume: Results of a Pilot Study. J Vasc Endovasc Surg. 2017, 2:16. doi: 10.21767/2573-4482.100048
Abstract
Introduction: In selecting patients for carotid revascularization, relying solely on symptoms and/or degree of stenosis is not sufficient. Patients with severe stenosis often remain asymptomatic, while other patients with moderate stenosis experience stroke. In contemporary practice, surgery or intervention is carried out much more often in asymptomatic patients. With the advent of improved medical management, the indications for surgery or intervention in the asymptomatic have become unclear. We utilized a technique for measuring Plaque Vasavasorum Volume (PVV) using Contrast Enhanced Carotid Duplex Ultrasound (CECDU) as a novel method for identifying asymptomatic patients with carotid artery stenosis who are at highest risk for stroke and thus more likely to benefit from early revascularization.
Objective: To establish the diagnostic accuracy and reliability of PVV measured by CE-CDU in identifying high risk carotid plaques and to identify a diagnostic cut-off point beyond which the risk of stroke exceeds the risk of surgery/intervention in asymptomatic patients with carotid stenosis.
Methods: The Stroke Prevention with Plaque Vasa-vasorum in Carotid Stenosis Study (SPIVICS) is a two phase multi-center double blinded prospective study. The first phase is a cross-sectional study of patients who qualify for carotid endarterectomy per current guidelines (asymptomatic patients with >70% stenosis and symptomatic patients with >50% stenosis). End points are analyzed using standard univariate and multivariate regression, correlation and diagnostic analyses.
Outcomes: Primary outcome relating to plaque morphology is the PVV volume. Clinical outcomes are neurologic symptoms (Amaurosis fugax, transient ischemic attack, and stroke) and asymptomatic events detected on imaging.
Results: A total of 16 patients were examined in this pilot study. Three were leadin subjects and four of them had severe calcified plaque and VV was not visualized. For those who had a successful screen, Mean age was 69 (± 7) years, Five males and the mean plaque VVV was 0.29 (± 0.62) mm.
Conclusion: Plaque Vasa-vasorum Volume is a promising predictor of stroke risk. By identifying patients who are truly at high risk for stroke, PVV measured by CECDU will establish more reliable criteria for patient selection for intervention. This in turn will help with stroke prevention, limit disability, save lives, and expend health care resources in a more selective manner.
Keywords
Carotid artery; Revascularization; Carotid stenosis
Introduction
Carotid artery stenosis remains a significant cause of stroke [1,2] and stroke is the third leading cause of death and the primary cause of serious, long-term disability in the United States [3-5]. The availability of advanced imaging modalities for detecting and quantifying asymptomatic carotid artery stenosis is of considerable interest in contemporary practice. Advancements in medical therapy heighten the need to identify patients with carotid stenosis whose risk of stroke clearly exceeds the risk of revascularization.
Current treatment modalities for prevention of stroke in patients with carotid artery stenosis include medical management, Carotid Endarterectomy (CEA), and Carotid Angioplasty and Stenting (CAS). In contemporary practice, patient selection is based on the severity of the stenosis and the occurrence of symptoms such as Amaurosis fugax, transient ischemic attack, or stroke. The indications for CEA are carotid stenosis greater than 50% in symptomatic patients or stenosis greater than 60% in asymptomatic patients [6-8]. Patients who are at high risk for surgery (CEA) might be considered for CAS [9].
Clinically, however, it is not infrequently observed that patients with severe stenosis remain asymptomatic while some patients with moderate stenosis experience stroke. This implies that the degree of stenosis may not be the most reliable predictor of stroke risk, but rather plaque instability may be a more sensitive metric [10]. The reliance on symptoms is also not desirable because Amaurosis fugax and Transient Ischemic Attacks (TIA) are not entirely benign. Furthermore, the majority of patients are asymptomatic until they experience a stroke. Among those who experience a TIA before a stroke, the symptoms not infrequently occur on the day or within 24 h of the stroke, affording the clinician very limited time to intervene. Therefore, it is essential to identify asymptomatic individuals who truly have an increased risk of subsequently becoming symptomatic. Additionally, the majority of CEA and CAS procedures are performed today for asymptomatic disease. A better method for identifying higher risk asymptomatic carotid plaques would allow for a more informed method of selecting patients for intervention. There is a critical need for an objective, non-invasive diagnostic tool beyond degree of stenosis that more accurately predicts plaque instability and thus the risk of stroke in asymptomatic patients.
Prior studies have demonstrated that patients with uns-atherosclerotic carotid plaques carotid plaques are at highest risk for stroke [10]. However, no diagnostic tool or technique in current use has the ability to predict with certainty the instability of plaque atheroma and the subsequent risk for stroke [11]. The evolution of plaque instability involves vasa-vasorum (VV), which is the network of micro-vessels that supply or drain the walls of larger arteries such as the carotid artery. By applying contrast to duplex ultrasound, we have developed a method for estimating stroke risk in asymptomatic patients by measuring the volume of plaque vasa-vasorum in the carotid arteries based on a priori hypothesis derived from the literature and clinical knowledge. Herein, we present the result of a pilot study aimed at establishing Plaque Vasa-vasorum Volume (PVV) measured by Contrast Enhanced Carotid Duplex Ultrasound (CE-CDU) as a valid and reliable diagnostic tool for predicting stroke risk in patients with asymptomatic carotid artery stenosis.
Methods
Design and patient population
The Stroke Prevention with Plaque Vasa-vasorum in Carotid Stenosis Study (SPIVICS) is a two phase multi-center double blinded prospective study. Study approval was granted by the Johns Hopkins institutional review board and all subjects gave written informed consent. The first phase is a cross sectional study of patients who qualify for carotid endarterectomy per current guidelines (asymptomatic with >70% carotid stenosis or symptomatic patients with >50% carotid stenosis). The second phase is a prospective study of asymptomatic patients with 50- 69% carotid stenosis. Adults with no child bearing potential or have negative pregnancy test within one week prior to enrollment with proper indication for CEA were included. Patients were excluded if they are having an evolving stroke, active bleeding or severe dementia. Further detailed inclusion and exclusion criteria are outlined in Table 1. All potential participants were evaluated for eligibility by the study vascular surgeon and coordinator.
| Inclusion criteria |
| • Age ≥ 18 years old. • Symptomatic status. • Symptomatic: Evidence of Transient Ischemic Attack (TIA), amaurosis fugax, minor or non-disabling stroke (in the hemisphere supplied by the target vessel), within 180 days of the date of enrollment. • Asymptomatic: Patients who do not meet the definition of symptomatic status. These include, patients with no prior carotid territory symptoms or, with symptoms arising from the hemisphere contralateral to the target vessel, symptoms in either hemisphere >180 days prior to the enrollement, or vertebrobasilar symptoms only. • No child bearing potential or has negative pregnancy test within one week prior to enrollment. • Written informed consent. • Lesion located in the carotid artery with or without involvement of the common carotid artery. |
| Exclusion criteria |
| • Evolving stroke. • Intolerance to contrast media. • Active bleeding diathesis or coagulopathy or will refuse blood transfusion. • Prior major ipsilateral stroke. • Severe dementia. • Spontaneous intracranial hemorrhage within 12 months. • Neurologic illness within past year characterized by fleeting or fixed neurologic deficit that cannot be distinguished from TIA or stroke. • Inability to understand and cooperate with study procedures or provide informed consent. • Chronic atrial fibrillation. • Other sources of cardiac emboli: Left ventricular aneurysm, cardiomyopathy, aortic or mitral prosthetic heart valve, calcific aortic stenosis, endocarditis, mitral stenosis, atrial septal defect, atrial septal aneurysm, left atrial myxoma. |
Table 1: Eligibility criteria.
Cross Sectional Phase
Contrast enhanced carotid duplex ultrasound
Consenting and eligible patients initially undergo standard carotid duplex ultrasound and stenosis is graded in accordance with the Society of Radiologists in Ultrasound Consensus (Table 2) [12]. After informed consent is obtained within 24 h of the scheduled CEA, contrast enhanced carotid duplex ultrasound (CE-CDU) is carried out with: OPTISON contrast Perflutren protein-type A microspheres injectable suspension, USP (General Electric Company, Fairfield, CT) and Philips IU22 ultrasound machine equipped with L9-3 linear probe (Philips Healthcare, Andover, MA). The recommended dose of OPTISON (0.5 mL) is injected into a 16-22 gauge or larger peripheral intravenous access line followed by a saline flush. Within 10-12 sec, contrast enhanced ultrasound images are obtained. If the contrast enhancement is inadequate after the initial dose of 0.5 mL, additional doses in increments of 0.5 mL may be repeated guided by the following protocol: (1) The injection rate will not exceed 1 mL per second, (2) Optison injection will be followed with a flush of 0.9% Sodium Chloride, USP, or 5% Dextrose, USP, (3) Maximum total dose will not exceed 5.0 mL in any 10 min period, and (4) Maximum total dose will not exceed 8.7 mL in any patient per study.
| Symptomatic | Asymptomatic | |
|---|---|---|
| ICA Stenosis | >50% | >70% |
| ICA Peak Systolic Velocity (PSV) | 126-230 cm/s | >230 cm/s |
| End Diastolic Velocity (EDV) | 40-99 cm/s | ≥ 100 cm/s |
| ICA/CCA PSV Ratio | 2.0-4.0 | ≥ 4.0 |
| ICA: Internal carotid artery; CCA: Common carotid artery | ||
Table 2: Society of radiologists in ultrasound consensus for quantifying degree of carotid stenosis.
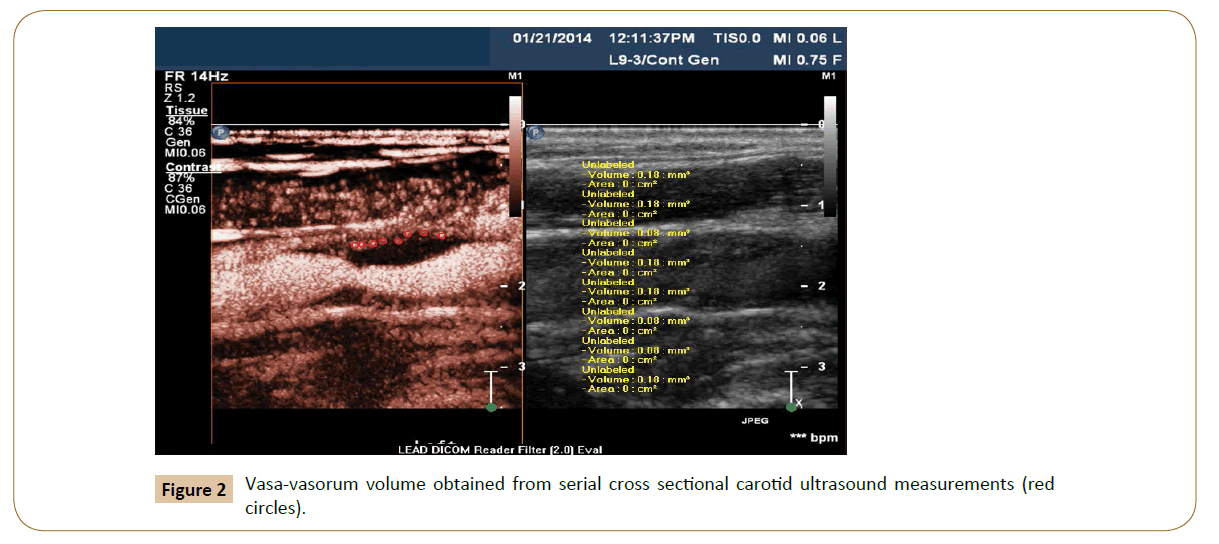
Two independent vascular specialists identify vasa-vasorum (Yes/ No) and take serial cross sectional measurements of PVV using Med-streaming Vascular Imaging Software. The bifurcation of the Common Carotid Artery (CCA) is considered the reference point for the measurements and all images and records are stored in Digital Imaging and Communications in Medicine format. For standardization, the volume of PVV measured is restricted to the region 5 mm proximal and 5 mm distal to the bifurcation of the CCA.
Carotid endarterectomy and histometric measurement of PVV in excised plaque
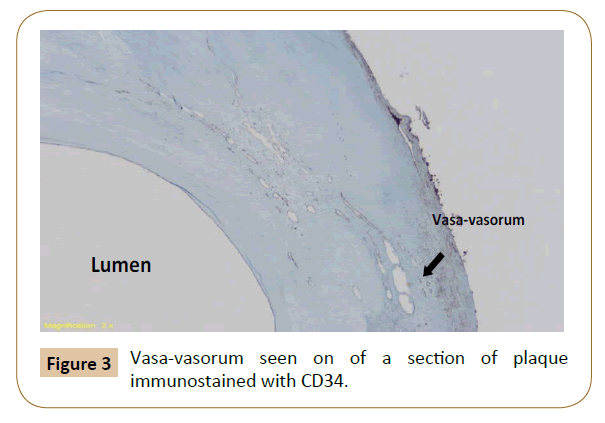
Patients undergo CEA per our routine standard of care. During CEA, the plaque is excised intact and marked at the bifurcation of the Common Carotid Artery (CCA) and its most proximal superficial point for orientation. The marked bifurcation of the CCA is regarded as the reference point for all measurements. The excised plaque is transported in formaldehyde to pathology core lab for histometric analyses. The plaque is decalcified using Rapid Cal-Immuno (BBC Biochemical, Seattle, WA). Subsequently, 3 mm cuts are made through the specimen starting from 1 cm proximal to 1 cm distal to the bifurcation of the CCA. Specimen sections are immunostained with CD-34 to identify vasavasorum. Cross sectional measurements of PVV are carried out and histometric volume is reconstituted by two independent pathologists.
Prospective phase
At baseline, the following procedures are carried out: (1) standard carotid duplex; (2) CE-CDU; (3) Transcranial Doppler (TCD). Discrete measurements of PVV using CE-CDU are obtained based on the protocol outlined in the cross sectional study. TCD is carried out in accordance with standard protocol using a “ROBOTOC2MD” digital transcranial ultrasound system (Multigon Industries INC, Yonkers, NY, USA) to detect embolic signals. Microembolic events are identified during 1 h long TCD recordings taken from the ipsilateral middle cerebral artery in accordance with the criteria of the Ninth International Cerebral Hemodynamic Symposium [13] and the recommendations of the International Consensus Group on Microembolus Detection [14].
These medically managed patients are routinely contacted every 3 months to ascertain the occurrence of symptoms (Amaurosis fugax, TIA, stroke). If asymptomatic, patients undergo standard carotid duplex ultrasound, CE-CDU, and TCDs semi-annually. If a symptom occurs, PVV is measured by CE-CDU and microembolic events are measured with TCD within 1 week of the event. For both cohorts, demographics, medical characteristics and medication profiles are recorded at baseline and through the entire study period.
Outcomes
In the cross sectional phase, the outcomes are the validity and reliability of PVV volume measured by CE-CDU in comparison to PVV volume measurements from histology as gold standard. In the prospective phase, the primary outcome is the occurrence of symptoms (Amaurosis fugax, TIA and stroke). This is based on the primary hypothesis that PVV volume measured by CE-CDU predicts the occurrence of ipsilateral symptoms in patients with carotid artery stenosis. Similarly, the secondary hypothesis is that PVV volume measured by CE-CDU increases in tandem with the occurrence of symptomatic neurologic events. As such, the secondary outcome is the occurrence of embolic signals on TCD.
Study duration
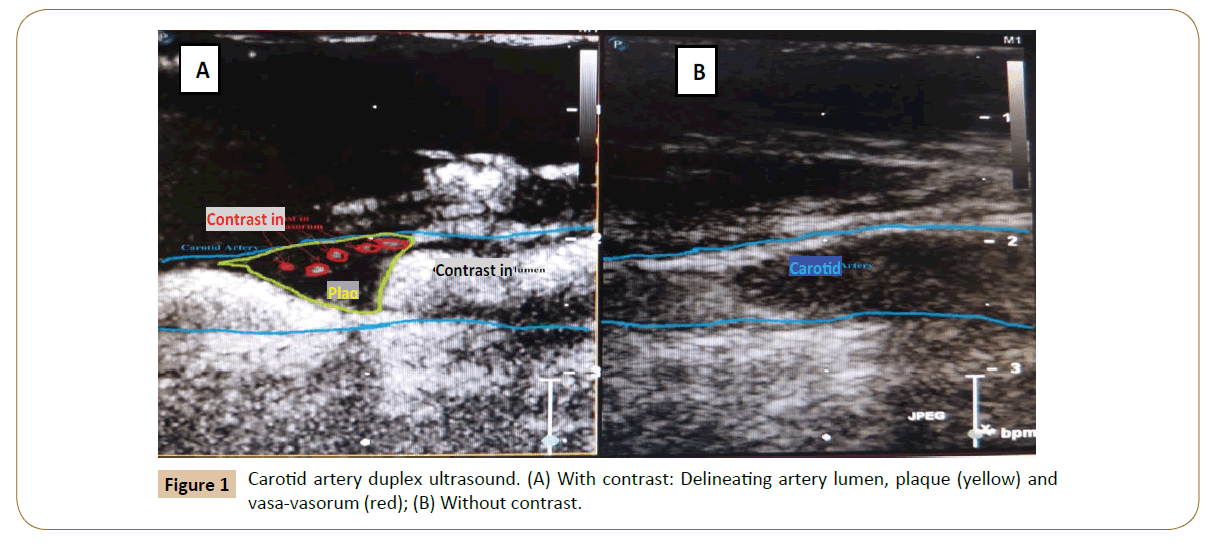
Enrollment into the cross-sectional phase commenced in October, 2013. Enrollment into the prospective cohort will begin in May 2016 with follow up anticipated to be carried out over 5 years (Figures 1-3).
Statistical analyses
In the cross sectional cohort, the reliability of CE-CDU is assessed by comparing measurements obtained by the two evaluators using Kappa, Bland-Altman and Pearson product-moment correlation analyses. The diagnostic accuracy of CE-CDU is ascertained in reference to histometry by computing measures of validity (sensitivity and specificity) as well as positive and negative predictive values. In order to detect a kappa for interclass agreement of 0.70 from a two tailed test with null=0.4, given 70% proportion of positive rankings, the required number of patients (N) =48. This is based on the goodness of fit formula provided by Donner and Eliasziw [15].
Results
During the enrollment period, sixteen patients were examined. Three of them were used as lead-in subjects. They were cardiac patients undergoing catheterization and we sought to validate the CE-CDU. Of the remainder, four patients had highly calcified plaques and we were unable to visualize the VV. Mean age for the screened patients was 69 years and five of them were males (55.6%). The average vasa-vasorum volume detected was 0.29 (± 0.62) mm3. The measured PVV via the CE-CDU ranged from (0.0-1.88 mm3). The histometry measurement yielded a very close range of (0.49-1.81 mm3) (Tables 3 and 4).
| Baseline characteristics | Values |
|---|---|
| Mean age (± SD), in years | 69 (± 7) |
| Gender | |
| Male Female |
5 (55.6%) 4 (44.4%) |
| Mean VVV, (± SD) in mm3 | 0.29 (± 0.62) |
Table 3: Baseline characteristics of the screened patients in the pilot study.
| Vasa-vasorum volume | Range (mm3); N=9 |
|---|---|
| CE-CDU | 0.00–1.88 |
| Histometry | 0.49–1.81 |
Table 4: PVV measurements obtained from CE-CDU and histometry in the pilot study.
Discussion
Several randomized trials have compared treatment modalities for carotid stenosis in the prevention of disease progression, stroke and death [16-21]. The investigators in these trials have consistently identified proper patient selection as the key to maximize benefits from each treatment modality. The Clinical Practice Guidelines of the Society for Vascular Surgery and other professional societies also recommend management of asymptomatic patients based on individual risk profiles [6,22,23]. The case for careful patient selection is supported by the fact that surgery/intervention is not free of complications. Carotid endarterectomy in asymptomatic patients is associated with risk of death (0.3%), stroke (2.3%) and myocardial infarction (2.3%) [20]. Guay and Ochroch in their meta-analysis demonstrated that [11] carotid endarterectomies are required to prevent one stroke in asymptomatic patients with high grade carotid artery stenosis [24].
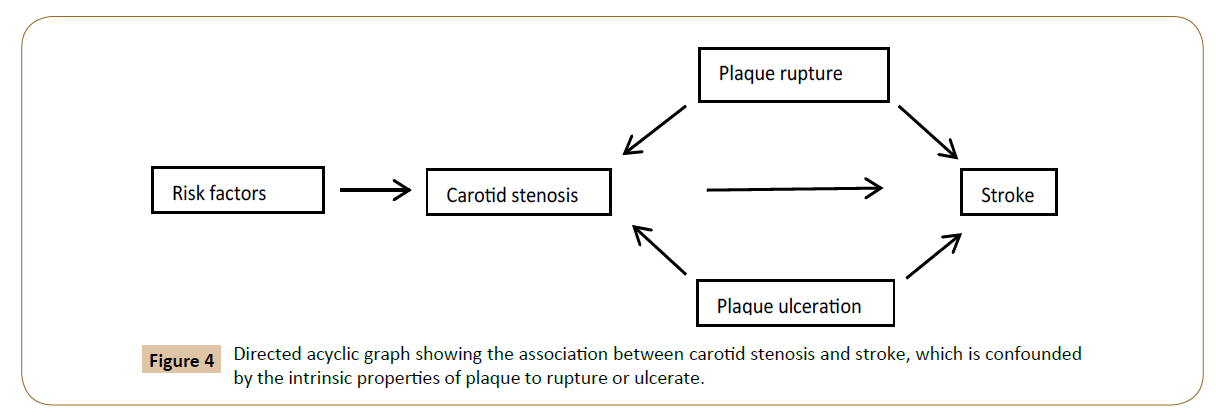
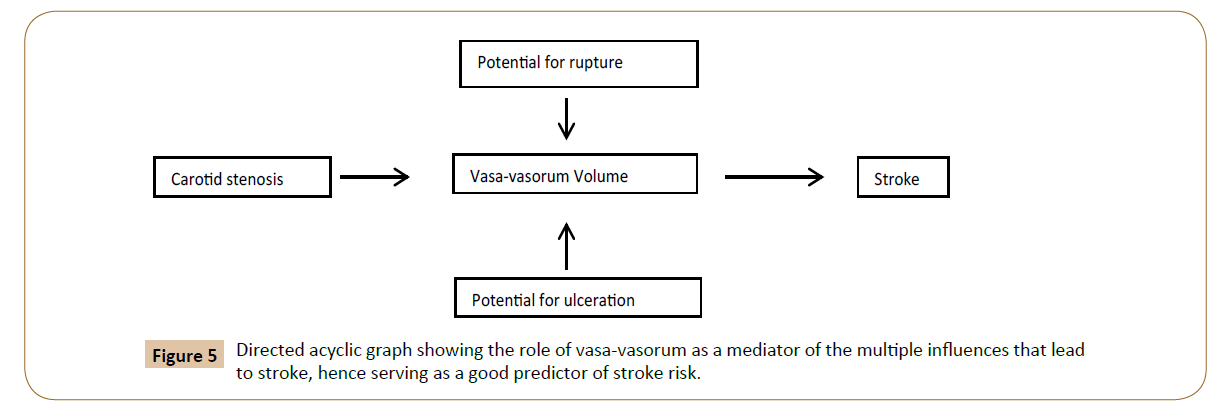
The shortcoming in the current modalities for diagnosis is the result of the confounded association between the degree of stenosis and stroke by intrinsic properties of plaque that predispose to rupture or ulceration (Figure 4). The framework for this project is based on the innovative concept that PVV mediates the association between degree of stenosis, potential to rupture/ ulcerate, and stroke (Figure 5).
The pathophysiological mechanisms for the development of carotid artery stenosis involve the accumulation of atheromatous plaque. This occurs under the influence of risk factors such as smoking, hypertension, diabetes and dyslipidemia that are the target of medical management for stroke prevention. The progression to an ischemic event from carotid artery stenosis commonly involves unstable plaque formation. The manifestation of certain cytokins such as FGF, TGF-B, TNF-a have been observed within atheromatic plaques and might accelerate the development of the plaque and its destabilization [25]. Unstable lesions had shown distinct protein expressions. For example, ST2L (Suppression of tumorigenity) was more expressed in membrane pattern of macrophages in symptomatic rather than asymptomatic plaques [26]. An unstable plaque is one that is at an increased risk of rupture because it consists of a thin fibrous layer overlying a necrotic lipid core. A novel proposed detection tool for the propensity of plaque rupture had been evaluated by Niccoli Asabella et al. via 18F-FDG positron emission tomography/computer tomography in which 18F-FDG uptake demonstrated inflammatory activation of plaque macrophages [27]. The evolution of plaque instability involves vasa-vasorum (VV). It has been shown that cellular hypoxia in the plaque prompts angiogenesis [28]. Adventitial VV hyperplasia and intimal neovascularization cause plaque instability. We believe that when vasa-vasorum volume reaches a critical point that exceeds fibrous cap strength, the plaque ruptures. At this point, the ruptured plaque may protrude into the lumen causing rapid progression to severe stenosis or complete occlusion at the region of stenosis.
Debris from the ruptured plaque might also travel to occlude distal cerebral small caliber vessels, causing ischemic brain injury. A second but less common mechanism of progression of carotid artery stenosis to stroke involves the formation of thrombus on ulcerated plaque. A significant correlation between ulcerated plaque and neovascularization has also been reported [29]. The unique presence of an intraplaque vessel under every ulcerated plaque points to neovascularization (plaque vasa-vasorum) as a key part of both pathways that lead to stroke. The fact that patients with unstable atherosclerotic plaques have the highest risk for stroke is also well known [10]. In this study, we present PVV as a novel surrogate for accurately predicting the instability of plaque atheroma.
Studies in animal models have shown that vasa-vasorum proliferate under the positive influence of risk factors [30]. In human studies, neovascularization in carotid and coronary plaques has been shown to increase in the presence of cardiovascular risk factors and regress when these factors are controlled [31]. In a recent study, vasa vasorum volume showed to predict progression of plaque volume in coronary arteries after heart transplantation [32]. However, no prior study has evaluated cerebrovascular disease progression based on objective and reproducible measurements of neovascularization using PVV in carotid arteries. Additionally, complex techniques such as 3D optical coherence tomography has been used to evaluate coronary adventitial vasa-vasorum [33] yet, to our knowledge, no previous study had adequately studied the role of the simple, cost-effective duplex ultrasound in measuring PVV.
CE-CDU is innovative in its use of contrast in carotid ultrasonography. In current practice, carotid ultrasonography involves standard imaging without contrast. Contrast is made up of micro bubbles that are approximately 1-8 μm in diameter. When introduced into the vessel, the contrast agent acts as a tracer because the size of the bubble approximates that of a red blood cell, rendering it trapped in the intravascular space. This property of contrast agents allows for the precise delineation of the arterial lumen, plaque atheroma, ulcerations and vasa-vasorum. Within minutes of administration, contrast is eliminated through the reticulo-endothelial system in the lungs. To date, there is paucity in the literature evaluating the precision of CE-CDU in measuring carotid PVV. For example, the authors of a recently published meta-analysis were able to identify only seven studies that report the sensitivity and specificity of CE-CDU and the reference tests varied among the studies included in the meta-analysis [34].
Studies on human coronary and carotid arteries have shown the ability of contrast-enhanced ultrasound to picture neovascularization [35-47]. These previous assessments of neovascularization were based on qualitative and subjective methods such as visual scoring and grading of signal enhancement only. No prior study has measured the volume of the PVV in carotid arteries. Neovascularization has been described as an initial phase that is characterized by hypertrophy of adventitial vasa-vasorum and a later phase that consists of proliferating new small vessels in the media and intima. This is the first study that is targeted at the objective quantification of neovascularization in carotid plaque based on vasa-vasorum volume (PVV).
Previous studies have shown that PVV can be identified on plaque atheroma excised from human carotid and coronary arteries [29,35-39]. However, the assessments of the degree of neovascularization were based on qualitative methods. These assessments were subjective and at best based on visual appreciation of the density of neovascularization or increased amount of immunostaining.
The occurrence of symptoms signifies disease that is advanced in its course such that a fatal or disabling stroke might occur. Therefore, in asymptomatic patients, it is desirable and indeed essential to identify those in whom progression of disease is likely and thus for whom intervention should be considered. We believe that the volume of vasa-vasorum increases in tandem with the progression to instability in plaque atheroma. Thus the measurement of PVV in asymptomatic patients with carotid stenosis will reliably indicate the stage of disease at which the risk of cerebrovascular events increases, thereby identifying those patients who would benefit most from surgery or intervention for stroke risk reduction.
The results of this investigation should be interpreted with caution since we are presenting the preliminary results of a prospective ongoing study. The conclusions are limited by the small sample size and the homogeneity of the institutional environment which might question the generalizability of the results to other settings until sufficient validation studies are available.
Conclusion
In this preliminary small pilot study, plaque vasa-vasorum volume is a promising potential predictor of stroke risk in patients with asymptomatic carotid artery stenosis. Contrast Enhanced Carotid Duplex Ultrasound (CE-3DCDU) is a safe, noninvasive, portable and inexpensive diagnostic tool for carotid artery stenosis. By identifying patients who are truly at high risk for stroke, PVV measured by CE-CDU has the potential to change clinical practice. This will enhance precise patient selection for intervention, thus maximizing benefits and reducing risk exposure in the fragile population of seniors, and reduce health care costs. This is a major step in the efforts to prevent stroke, save lives, expend health care resources in a more informed and selective manner, and limit the burden of disability in this era.
References
- White H, Boden-Albala B, Wang C (2005) Ischemic stroke subtype incidence among whites, blacks, and Hispanics: The northern Manhattan study. Circulation pp: 1327-1331.
- Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, et al. (2008) Carotid plaque, a subclinical precursor of vascular events: The Northern Manhattan Study. Neurology 70: 1200-1207.
- Minino AM, Murphy SL, Xu J, Kochanek KD (2011) Deaths: final data for 2008. Natl Vital Stat Rep 59: 1-126.
- Centers for Disease Control and Prevention (CDC) (2005) Prevalence of stroke-United States. MMWR Morb Mortal Wkly Rep 56: 469-674.
- Centers for Disease Control and Prevention (CDC) (2009) Prevalence and most common causes of disability among adults-United States, 2005. MMWR Morb Mortal Wkly Rep 58: 421-426.
- Brott TG, Halperin JL, Abbara S (2011) 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task F. J Am Coll Cardiol 57: e16-e94.
- Ritter JC, Tyrrell MR (2013) The current management of carotid atherosclerotic disease: Who, when and how? Interact Cardiovasc Thorac Surg 16: 339-346.
- Kernan WN, Ovbiagele B, Black HR (2014) Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 45: 2160-2236.
- Yadav JS, Wholey MH, Kuntz RE (2004) Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 351: 1493-1501.
- McCarthy M, Loftus I, Thompson M (1999) Vascular surgical society of great britain and ireland: angiogenesis and the atherosclerotic carotid plaque: association between symptomatology and plaque morphology. Br J Surg 86: 707-708.
- Ten Kate GL, Van Den Oord SCH, Sijbrands EJG (2013) Current status and future developments of contrast-enhanced ultrasound of carotid atherosclerosis. J Vasc Surg 57: 539-546.
- Grant EG, Benson CB, Moneta GL (2003) Carotid Artery StenosisÃÆâÃâââ¬Ãâï: Gray-Scale and Doppler US Diagnosis-Society of Radiologists in Ultrasound. Radiology 229: 340-346.
- The Consensus Committee of the Ninth International Cerebral Hemodynamics Symposium (1995) Basic identification criteria of Doppler microembolic signals. Stroke 26: 1123 LP-1123.
- Markus HS, Ackerstaff R, Babikian V (1997) Intercenter agreement in reading Doppler embolic signals. A multicenter international study. Stroke 28: 1307-1310.
- Donner A, Eliasziw M (1992) A goodness-of-fit approach to inference procedures for the kappa statistic: confidence interval construction, significance-testing and sample size estimation. Stat Med 11: 1511-1519.
- The Advisory, Conciliation and Arbitration Service (ACAS) (1995) Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. J Am Med Assoc 273: 1421-1428.
- Barnett HJ, Taylor DW, Eliasziw M (1998) Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 339: 1415-1425.
- Godwin J (1998) Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351: 1379-1387.
- Halliday A, Harrison M, Hayter E (2010) 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet 376: 1074-1084.
- Brott TG, Hobson RW, Howard G (2010) Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 363: 11-23.
- Hassoun HT, Malas MB, Freischlag JA (2010) Secondary stroke prevention in the era of carotid stenting: update on recent trials. Arch Surg 145: 928-935.
- Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, et al. (2011) Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg 54: e1-31.
- Liapis CD, Bell SPRF, Mikhailidis D (2009) ESVS guidelines. invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg 37: 1-19.
- Guay J, Ochroch EA (2012) Carotid endarterectomy plus medical therapy or medical therapy alone for carotid artery stenosis in symptomatic or asymptomatic patients: A meta-analysis. J Cardiothorac Vasc Anesth 26: 835-844.
- Janczak D, Ziolkowski P, Garcarek J, Janczak D, Dorobisz K, et al. (2014) The cytokines within the carotid plaque in symptomatic patients with internal carotid artery stenosis. J Cardiothorac Surg.
- Marzullo A, Ambrosi F, Inchingolo M (2016) ST2L Transmembrane Receptor Expression: An Immunochemical Study on Endarterectomy Samples. PLoS One 11: e0156315.
- Niccoli Asabella A, Ciccone MM, Cortese F (2014) Higher reliability of 18F-FDG target background ratio compared to standardized uptake value in vulnerable carotid plaque detection: a pilot study. Ann Nucl Med 28: 571-579.
- Sluimer JC, Gasc JM, van Wanroij JL (2008) Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol 51: 1258-1265.
- Vicenzini E, Giannoni MF, Puccinelli F (2007) Detection of carotid adventitial vasa vasorum and plaque vascularization with ultrasound cadence contrast pulse sequencing technique and echo-contrast agent. Stroke 38: 2841-2843.
- Schinkel AFL, Krueger CG, Tellez A (2010) Contrast-enhanced ultrasound for imaging vasa vasorum: Comparison with histopathology in a swine model of atherosclerosis. Eur J Echocardiogr 11: 659-664.
- Feinstein SB (2006) contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol 48: 236-243.
- Park KH, Kwon TG, Matsuzawa Y (2016) Association between the vasa vasorum and the atherosclerotic changes in cardiac allograft vasculopathy: volumetric analysis. Eur Hear J Cardiovasc Imaging 17: 272-279.
- Aoki T, Rodriguez-Porcel M, Matsuo Y (2015) Evaluation of coronary adventitial vasa vasorum using 3D optical coherence tomography–animal and human studies. Atherosclerosis 239: 203-208.
- Huang R, Abdelmoneim SS, Ball CA (2016) Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J Am Soc Echocardiogr 29: 491-502.
- Shah F, Balan P, Weinberg M (2007) Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med 12: 291-297.
- Coli S, Magnoni M, Sangiorgi G (2008) Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries. J Am Coll Cardiol 52: 223-230.
- Giannoni MF, Vicenzini E, Citone M (2009) Contrast Carotid Ultrasound for the Detection of Unstable Plaques with Neoangiogenesis: A Pilot Study. Eur J Vasc Endovasc Surg 37: 722-727.
- Shalhoub J, Monaco C, Owen DRJ (2011) Late-phase contrast-enhanced ultrasound reflects biological features of instability in human carotid atherosclerosis. Stroke 42: 3634-3636.
- Hoogi A, Adam D, Hoffman A, Kerner H, Reisner S, et al. (2011) Carotid plaque vulnerability: Quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. Am J Roentgenol 196: 431-436.
- Huang PT, Huang FG, Zou CP (2008) Contrast-enhanced sonographic characteristics of neovascularization in carotid atherosclerotic plaques. J Clin Ultrasound 36: 346-351.
- Magnoni M, Coli S, Marrocco-Trischitta MM (2009) Contrast-enhanced ultrasound imaging of periadventitial vasa vasorum in human carotid arteries. Eur J Echocardiogr 10: 260-264.
- Papaioannou TG, Vavuranakis M, Androulakis A (2009) In-vivo imaging of carotid plaque neoangiogenesis with contrast-enhanced harmonic ultrasound. Int J Cardiol 134.
- Xiong L, Deng YB, Zhu Y, Liu YN, Bi XJ (2009) Correlation of Carotid Plaque Neovascularization Detected by Using Contrast-enhanced US with Clinical Symptoms. Radiology 251: 583-589.
- Staub D, Patel MB, Tibrewala A (2010) Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke 41: 41-47.
- Owen DR, Shalhoub J, Miller S (2010) Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrast-enhanced US. Radiology 255: 638-644.
- Staub D, Partovi S, Schinkel AFL (2011) Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology 258: 618-626.
- Clevert DA, Sommer WH, Helck A, Saam T, Reiser M (2011) Improved carotid atherosclerotic plaques imaging with contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc 48: 141-148.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences