Coronary-Subclavian Steal Syndrome: Case Series and Review of Therapeutic Aspects
José Maciel Caldas dos Reis
DOI10.21767/2573-4482.20.05.16
Division of Vascular Surgery, Amazonia Hospital, Belém, Pará, Brazil
- *Corresponding Author:
- José Maciel Caldas dos Reis
Division of Vascular Surgery
Amazonia Hospital, Belém, Pará, Brazil
Tell: +559130845482
E-mail: macielreis.angiovasc@gmail.com
Received Date: June 30, 2020; Accepted Date: July 14, 2020; Published Date: July 21, 2020
Citation: Jose MCD Reis (2020) Coronary- Subclavian Steal Syndrome: Case Series and Review of Therapeutic Aspects. J Vasc Endovasc Ther. Vol.5 No.3:16.
Abstract
Background: Coronary-subclavian steal syndrome (CSSS) is a clinical condition characterized by the reversal of blood flow in the left internal thoracic artery (ITA) in patients who have undergone coronary revascularization using this artery. It is a rare cause of myocardial ischemia subsequent to stenosis or occlusion of the subclavian artery (SA) proximal to ITA coronary bypass. Proximal subclavian artery (SA) stenosis is present and atherosclerotic disease is the underlying pathophysiologic mechanism in the majority of cases. We report a series of patients with late CSSS treated through an endovascular approach.
Methods: The clinical data of 4 consecutive patients with CSSS who had undergone subclavian artery stenting between 2015 and 2019 were reviewed. The anatomic and clinical-angiographic characteristics of the series were considered. Follow-up was performed and a review of the therapeutic aspects is provided.
Results: From January 2015 to December 2019, 4 patients with CSSS were treated; 3 had stable and 1 unstable angina. Of the 4 patients, 3 had left SA stenosis (2 ostial to the origin and 1 in the middle segment), 1 had proximal occlusion of the left SA. Arterial access was at the brachial artery through surgical exposure. In 1 case of proximal occlusion of the left SA, simultaneous femoral and percutaneous brachial access was necessary. Predilatation of the stenotic lesion was performed in all cases. Balloon expandable stents were used in all patients with proximal ostial stenosis or occlusion and self-expandable stents in 1 with nonostial lesion. No patients developed angina during the follow-up period (12+4 months).
Conclusions: Endovascular therapy with angioplasty and subclavian artery stenting is a treatment of choice for CSSS, due to the high success rates, minimally invasive procedure, and low morbidity and mortality rates. This condition should always be suspected in patients with a history of myocardial revascularization, clinical angina, and asymmetry between upper limb pulses.
Keywords
Coronary-subclavian steal syndrome; Coronary bypass; Internal mammary artery; Subclavian stenosis; Myocardial ischemia
Introduction
The coronary-subclavian steal syndrome (CSSS), first described in 1974 by Harjola and Valle [1], develops in 0.5% to 4% of patients undergoing myocardial revascularization (MR). Usually refers to a reversed blood flow from the myocardium to the upper limb due to ipsilateral subclavian artery stenosis (SAS) in patients with previous revascularization using the internal thoracic artery (ITA) [2,3].
The use of left ITA for coronary artery revascularization has been associated with better long-term patency and patient survival than the use of a saphenous venous graft because in situ has superior patency rate and survival benefit when grafted to the left anterior descending artery [3-5]. It is well known that each subclavian artery (SA) has four main branches, the ITA being one of them [3-6]. The use of the ITA is better associated with long-term MR surgery due to being a conduit usually spared from atherosclerosis [4-6]. However, this is not true for the proximal (“in flux”) part of this conduit, the subclavian artery, where atherosclerotic stenosis can affect the vessel [3-6]. Atherosclerosis represents more than 90% of subclavian artery stenosis [4-6]. Less common etiologies include arteritis (for example, Takayasu’s arteritis, giant cell arteritis), inflammation, radiation exposure, compression syndromes, fibromuscular dysplasia, and neurofibromatosis [5,6].
CSSS should be suspected in patients undergoing revascularization using the ITA with different pulse and blood pressure in the upper limbs, and clinical presentations of angina [4,7,8]. Significant stenosis or even occlusion of the subclavian artery proximal to the ITA can lead to decreased blood flow to the upper limb, triggering a flow inversion in the ITA with a concomitant hemodynamic “steal” of coronary blood flow to the upper limb [7-10]. CSSS patients usually present stable or unstable angina and may also present arm claudication and several neurological symptoms8-10. CSSS consequences can include ischemic cardiomyopathy, acute myocardial infarction (AMI), cerebrovascular accident, and death. CSSS is treated by correcting the stenosis or occlusion of the subclavian artery [3,6,7].
This study describes the cases of four patients with CSSS with the following clinical presentations: three with stable angina and one with unstable angina. In addition, a review of the therapeutic aspects is provided.
Methods
The experience of a vascular surgery center (Heart Hospital, Belém, Pará, Brazil) in the treatment of CSSS was retrospectively reviewed from January 2015 to December 2019. All patients signed an informed consent form for the procedure. The study followed the rules of the institution’s ethics committee. All patients undergoing MR surgery with an ITA graft and presenting the clinical symptoms of angina were evaluated by cardiac catheterization and supra-aortic angiography. Some patients also underwent arterial computed tomography angiography of the chest. Stenosis treatment was indicated due to the simultaneous presence of symptoms of myocardial ischemia and significant stenosis of the subclavian artery proximal to the origin of the ITA.
Patients and Procedures
The patients’ clinical characteristics, interval between MRI surgery and new symptoms of angina, type of myocardial symptoms, and technical aspects of the revascularization procedure were registered in a dedicated database. Stenosis was categorized as ostial or located in the middle segment of the subclavian vessel proximal to the ITA. Clinical characteristics included: age, sex, hypertension, dyslipidemia, diabetes, current smoking habits, and chronic renal failure.
All procedures were performed with patients under local anesthesia and systemic heparinization. Access site varied according to the location and type of occlusive disease (stenosis versus occlusion). In vascular surgery service, the approach of choice was through the brachial artery. When necessary, predilation was performed to cross the stenosis with the stent.
Balloon expandable stents were used for ostial lesions and selfexpanding stents for non-ostial lesions. Perioperative results included “technical success”, defined as a treatment resulting in < 30% residual stenosis.
Case Series
Case 1: Unstable angina
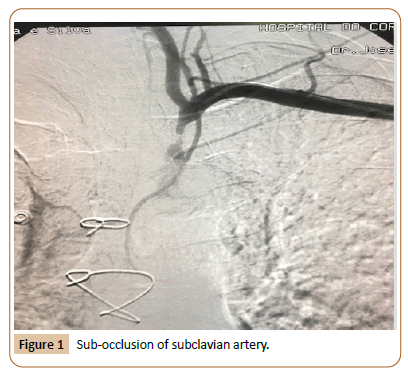
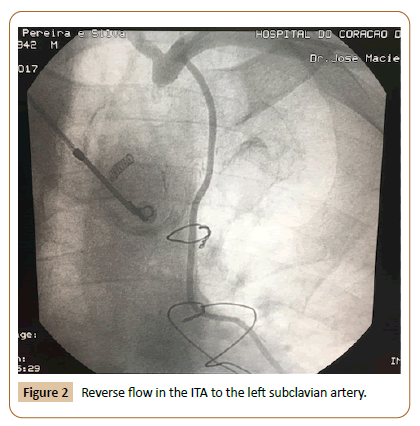
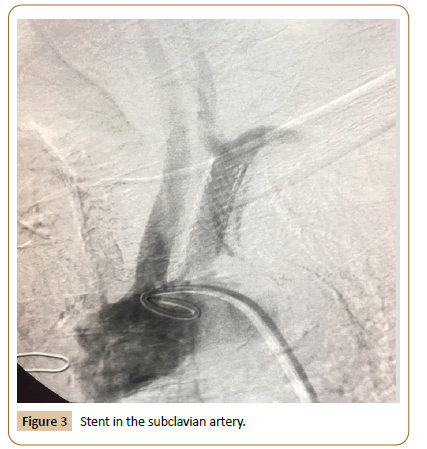
Male patient, 69 years old, ex-smoker, with a history of hypertension, dyslipidemia and diabetes. In 2012, he underwent coronary revascularization after acute myocardial infarction, using the left ITA to revascularize the anterior descending artery and the internal saphenous vein to revascularize the marginal oblique artery. In January 2017, the patient presented retrosternal chest pain with dorsal irradiation with relieve at rest. He was admitted to an intensive care unit with a diagnosis of acute coronary syndrome due to recurrent pain at hospitalization. The analytical study showed increased myocardial necrosis markers, without increased ST segment on the electrocardiogram (ECG). Physical examination showed absence of axillary, brachial, radial, and ulnar pulse in the left upper limb, with significantly different blood pressure in the upper limbs (130/70 mmHg on the right and 80/60 mmHg on the left). The patient presented no supraclavicular or carotid artery murmur, and the arterial pulses in the right upper limb were normal. Myocardial scintigraphy showed an ischemic area on the anterior wall. Cardiac catheterization identified permeability in both bridges, absence of new lesions in the coronary arteries, ostial occlusion of the left subclavian artery (Figure 1), and reverse ITA flow to the left subclavian artery (Figure 2). Thus, the patient was diagnosed with CSSS. Doppler examination of the carotid and vertebral arteries also showed flow inversion in the left vertebral artery. Considering this diagnosis, endovascular therapy was proposed for left subclavian artery occlusion. Thus, a guidewire was passed through the left brachial and right femoral access, the collusion was subsequently pre-dilated, and an 8.0 x 30 mm balloon expandable stent was implanted (Figure 3).
Case 2: Stable angina
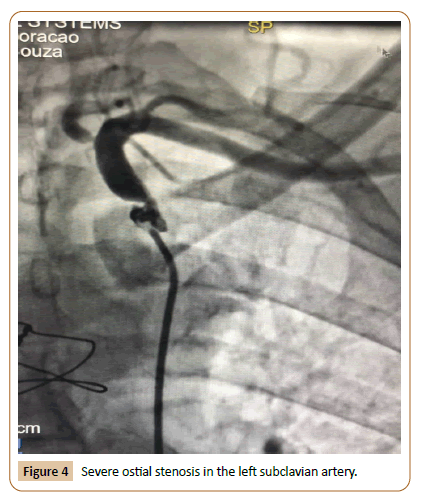
Female patient, 77 years old, hypertensive, diabetic and smoker, admitted for stable angina and MR surgery 7 years before. She presented exercise-induced angina and anterior wall ischemia on the ECG, but without increased cardiac biomarkers. On physical examination, there was a significant difference in blood pressure between the right and left arms (130/80 mmHg and 90/60 mmHg, respectively). Transthoracic echocardiogram showed a 42% reduction in the left ventricular ejection fraction (LVEF). A cardiac catheterization performed 24 hours after admission showed patent saphenous bypass grafts. However, a severe ostial stenosis in the left subclavian artery was diagnosed and considered responsible for the clinical complaint (Figure 4). Heart failure therapy was optimized, and the patient underwent balloon angioplasty for pre-dilation followed by the release of an 8.0 x 30 mm balloon expandable stent in the left subclavian artery. During follow-up, the left ventricular systolic function recovered to normal values.
Case 3: Stable angina
Female patient, 68 years old, hypertensive, diabetic, and under chronic renal dialysis due to arteriovenous fistula in the right upper limb. The patient underwent MR surgery 7 years before (3 bridges). Moderate aortic valve stenosis, progressing with recurrent symptoms of stable angina. On physical examination, the patient was comfortable with a heart rate of 75 bpm, blood pressure of 120/80 mmHg (measured on the right arm, with no significant blood pressure difference on the left side). Her peripheral pulses were full and symmetrical in the lower extremities, and no peripheral edema was observed. Admission ECG was not found in the patient’s medical record. Echocardiogram showed no significant changes. She underwent a stress test to verify a possible coronary distribution for the cause of the chest pain. Afterwards, a cardiac catheterization showed 95% non-ostial stenosis in the left subclavian artery. The patient underwent successful balloon angioplasty for pre-dilation, followed by the release of an 8.0 x 25mm self-expanding stent in the subclavian artery. After the procedure, the patient remained asymptomatic during an eight-month follow-up.
Case 4: Stable angina
Male patient, 71 years old, with hypertension, dyslipidemia, and type 2 diabetes mellitus. The patient underwent myocardial revascularization surgery 8 years before using an ITA graft in the anterior descending artery and two other saphenous vein aortocoronary grafts. The patient started presenting recurrent angina, which worsened 30 days before admission. Physical examination showed minor signs of anemia and a difference in systolic blood pressure of 30 mmHg between the upper extremities (full right radial pulse and filiform left pulse). No murmur was detected in the left subclavian or carotid region. ECG was suggestive of ischemia in the anterior wall of the myocardium and echocardiogram showed decreased left ventricular ejection fraction (LVEF=44%) with anterior wall hypokinesia. Carotid/ vertebral artery color doppler detected a typical image of vertebral flow inversion. The patient underwent cardiac catheterization, showing patency of the two venous grafts, but significant ostial stenosis (90%) of the left subclavian artery, which was considered the cause of the clinical findings. Angioplasty was performed with an 8.0mm x 30mm balloon-expandable stent implant. Poststent angiography showed recovered anterograde flow in the left vertebral artery and excellent ITA flow. After the procedure, the patient remained without anginal complaints.
Results
General characteristics
From January 2015 to December 2019, 4 CSSS patients were treated; 3 with stable angina and 1 with unstable angina. Of the 4 patients, 3 had subclavian artery stenosis (2 ostial at the origin and 1 in the middle segment), and 1 had proximal occlusion of the left subclavian artery. The clinical-angiographic characteristics of the patients are described in Table 1. The access of choice was the brachial artery. In 1 case of proximal occlusion of the left subclavian artery, simultaneous femoral and percutaneous brachial access was necessary. All cases underwent pre-d ilation of the stenotic lesion. Balloon-expandable stents were used in all patients with stenosis or proximal ostial occlusion, and a self-expanding stent was used in 1 case with non-ostial lesion in the left subclavian artery. No patient developed angina during the follow-up period (12+4 months). The patient with unstable angina had total ostial occlusion of the left subclavian artery. Technical success was achieved in all 4 patients. There were no complications at the puncture site and no clinical cardiac or neurological presentations developed during surgery or in the immediate postoperative period. No significant hematoma was observed postoperatively. All procedures were performed with ultrasound-guided puncture. At a mean follow-up of 12+4 months, all patients were asymptomatic for new anginal symptoms, without indirect signs of restenosis on duplex ultrasound performed on the limb.
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Sex | M | F | F | M |
| Age (years) | 69 | 77 | 68 | 71 |
| Type of lesion | ||||
| Stenosis | N | Y | Y | Y |
| Obstruction | Y | N | N | N |
| Lesion site | ||||
| Ostial | Y | Y | N | N |
| Non-ostial | N | N | Y | N |
| Type of stent | ||||
| Self-expanding | N | N | Y | N |
| Balloon expandable | Y | Y | N | Y |
| Comorbidities | ||||
| Hypertension | Y | Y | Y | Y |
| Diabetes | Y | Y | Y | Y |
| Dyslipidemia | Y | N | Y | Y |
| Smoking | N | Y | N | N |
| CRF | N | N | Y | N |
| Interval between MR surgery and symptoms (years) |
5 | 7 | 7 | 8 |
M (Male); F (Female); Y (Yes); N (No); CRF (Chronic Renal Failure); MR
(Myocardial Revascularization)
Discussion
A rare cause of recurrent myocardial ischemia, CSSS was first described by Hargola and Valle in 1974 [1], when they reported a case of reverse ITA flow caused by proximal occlusion of the left subclavian artery [1]. In complete obstructions, the ITA flow can be basically reversed, causing persistent circulatory steal in the anterior descending artery region [4,5].
ITA is the most used graft to restore coronary circulation, as it has been associated with better long-term patency and patient survival than with use of saphenous vein graft [4-9]. Some factors contribute to this durability, such as its functional endothelium, which produces potent vasodilators and platelet function inhibitors, and the continuous elastic lamina that inhibits smooth muscle migration and, therefore, arteriosclerosis [5,7,10,11]. During MR surgery, the proximal end of the ITA is connected to the left subclavian artery while the distal end is dissected and anastomosed to the diseased coronary artery [5]. CSSS typically results from hemodynamically significant stenosis of the subclavian artery proximal to the ipsilateral ITA [12]. The clinical spectrum of CSSS, depending on the hemodynamic impact of the lesion, includes exercise-induced angina, silent ischemia or even AMI with or without ST-segment increase [3,11,12].
Classic CSSS signs and symptoms include: angina typically related to homolateral upper limb activity, acute coronary syndromes like unstable angina or AMI, vertebrobasilar insufficiency symptoms, supraclavicular murmurs, homolateral arm claudication and coldness, brachial pulse asymmetry and different pressure between the two arms [6-10,13]. Thus, in revascularized patients presenting with acute or insidious myocardial ischemia, we always remember the CSSS as a possible etiology.
The dangerous nature of this syndrome has been acknowledged and can be associated with cerebrovascular disease due to ipsilateral vertebral artery flow inversion. The case series presented in this study showed no stable or unstable angina symptoms associated with vertebrobasilar insufficiency, such as neurological deficits, vertigo, fainting or ophthalmological disorders [11-14].
Different strategies have been suggested, including pressure gradient assessment between the upper extremities as an initial step; CSSS should be considered when the pressure gradient is >20 mmHg. Other strategies include duplex ultrasound scanning with provocation test using arm exercise or reactive hyperemia [12,14-17].
Thus, it is essential to screen subclavian artery disease before MR surgery. The left SA is the branch of the aortic arch most affected by atherosclerosis [3-6]. This explains why the immense majority of CSSS cases in the literature occur on the left side. Bilateral blood pressure measurement is the simplest and most economical screening method for significant subclavian artery stenosis, but it may not be identified in patients with bilateral subclavian artery stenosis [16,17]. All patients with a history of MR surgery must undergo an objective and complete physical examination, including systolic blood pressure measurement in the upper limbs, wrist palpation, and supra and infraclavicular auscultation [17]. In case of significant changes, diagnostic confirmation tests may be necessary, such as echocardiography, computed tomography angiography, and angiography [11,18].
The onset of ITA reverse flow when injecting into the left coronary artery is the angiographic sign that defines the CSSS [17]. Preventive measures include the adequate preoperative assessment of claudication symptoms in the arms, brachial pulse symmetry, supraclavicular auscultation, and arterial pressure in both arms. If any finding suggests subclavian occlusive disease, it is mandatory to perform a complementary imaging exam during the diagnostic study [2-4,6]. Although the systematic performance of selective ITA or aortic arch angiography in preoperative diagnostic catheterization is recommended by some authors, in general it has proved to be unnecessary and may even result in inadvertent ITA lesion [3-6,17-19]. Most authors agree that an adequate clinical evaluation is enough to select patients to undergo aortic arch branch angiography [5-7,17-20]; others recommend it in patients with diffuse arteriosclerosis, even in the absence of compatible signs or symptoms [21,22].
Although conventional surgical reconstruction was previously considered the first choice for CSSS management, it was gradually replaced by endovascular therapies due to lower complications rates and equal efficacy in short and medium follow-up periods reported in recent studies [11-13,16-20]. Thus, surgical correction is the method of choice when anatomy is not favorable to percutaneous intervention, such as in chronic total occlusion [17]. However, CSSS can be effectively managed in a less invasive manner with percutaneous intervention, including angioplasty with balloon catheter and stent in the subclavian artery. The advantages are the use of local anesthesia, less peri-procedure morbidity and mortality, and shorter hospital stay [3,5,12,20].
The most recent guidelines from the European Society of Cardiology and the American Heart Association recommend percutaneous balloon angioplasty with stent support as the firstline treatment [16]. In the last decades, endovascular subclavian artery therapy showed high technical success rates (97%) and excellent long-term permeability rates (89%-95%, after 5 years), with faster recovery, shorter hospital stay, lower morbidity and mortality (4.5%), and low complication rates (2.3% to 3.6% combined risk of ischemic cerebrovascular accident and death) compared to conventional surgery [16]. Thus, the interventionist technique with stent implantation became the method of choice to correct the CSSS [5,8,12,17,20]. As in all comparisons between percutaneous endovascular procedures and surgical revascularization, the primary patency rate was lower with endovascular techniques, but improved dramatically with the introduction of stent support and balloon angioplasty [4-6], with long-term outcome estimates of 78% stent permeability after 10 years [5,16,20]. However, conventional surgical correction is still the approach to be used if it is not possible to transpose the lesion during angioplasty in long or very calcified occlusive lesions with significant risks of cerebral circulation, upper limb or even ITA embolization [11,17,19,21,22].
The choice of balloon-expandable stents instead of selfexpanding stents in most cases (3 out of 4 cases) was due to their greater radial strength and precision during release. However, in very significant lesions or occlusions, pre-dilation was used to facilitate the passage of the stent release system and prevented it from being deformed on the balloon. Balloon-expandable stents were used in two cases in oue series, because ostial lesion due to the need of high implantation precision to avoid unintentional stent protrusion into the aortic arch or distal sliding. However, the self-expanding stent was the option for stenosis in the nonostial segment of the artery due to the need for lower radial force and the low risk of slipping. In the cases presented, two patients required a pre-dilatation due to severe stenosis or occlusion.
Post-angioplasty thrombosis is rare; however, long-term intra-stent stenosis was described by Schilliner et al. [23] as relatively frequent, reaching estimates of up to 40% in five years. Nevertheless, the comparison between stent angioplasty and open surgical treatment shows that the procedures have comparable effectiveness, with fewer complications and morbidity in the angioplasty group, which makes the procedure eligible for most cases [23,24]. In this series, coronary steal occurred between 5 and 8 years after MR (on average, 6.7 years). This suggests that these SA occlusive lesions most likely developed after left ITA grafting.
Several reports and case series have been described showing the personal experience of groups with a specific technique, but the literature still lacks prospective, randomized studies comparing percutaneous endovascular techniques with surgical revascularization of the SA. One of the largest consecutive cases series was published by Che et al. In 2017 [20], reporting low perioperative and follow-up complication rates in 37 patients undergoing angioplasty and stenting in the SA [20].
However, absence of any occlusion during follow-up (12+4 months) in our small series, suggests that endovascular management of SA steno-occlusive desease may be an excellent alternative to surgical bypass grafting. On the other hand, an adequate surveillance program can be established, enabling early detection and treatment of any significant recurrent stenosis, with comparable long-term patency rates.
The study has some limitations, as it is a retrospective analysis of a limited number of patients in a cardiological reference center in which many patients are from different regions of the state, making follow-up more difficult. As the screening test for subclavian artery lesions is not performed routinely, the current CSSS incidence is not available in the studied institution. A longer follow-up is ideal and can show data that could change the initial conclusions of this study.
Conclusion
CSSS is a serious and silent pathological entity resulting from the extensive atherosclerotic involvement of the left subclavian artery. The clinical spectrum of the syndrome includes silent ischemia, anginal symptoms, or even AMI, depending on the local hemodynamics of the lesion. Its prevalence is underestimated and duplex ultrasound, computed tomography, or angiography of the subclavian artery should be considered in all patients with suggestive clinical findings. Restoring the anterograde ITA flow is the key to preserve coronary perfusion. Currently, the endovascular approach is the technique of choice due to its high success rates, low morbidity, and mortality and for being minimally invasive.
Conflicts of Interests
None
Financial Support
None
References
- Harjola PT, Valle M (1974) The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest 66: 436-438.
- Tariq S, Tuladhar S, Wingfield E, Poblete H (2012) Coronary subclavian steal syndrome unamenable do angioplasty successfully managed with subclavian-subclavian bypass. Case Rep Vascul Med 2: 784231.
- Marc M, Iancu A, Molnar A, Bindea D (2015) Coronary-subclavian steal: Case series and review of the literature. Clujul medical 88: 79-82.
- Vogel JHB, Kristen A, Kloos W, Kohler B, Katus HA, et al. (2017) Interventional treatment of the left subclavian in 2 patients with coronary steal syndrome. World J Cardiol 9: 65-70.
- Cua B, Mandani N, Halpin D, Jhamnani S, Jayasuriya S, et al. (2017) Review of coronary subclavian steal syndrome. J Cardiol 70: 432-437.
- Abdul Jabbar A, Houston J, Burket M, Il'Giovine ZJ, Srivastava BK, et al. (2017) Screening for subclinical subclavian artery stenosis before coronary artery bypass grafting: Should we do it? Echocardiogr 34: 928-933.
- Cwinn M, Nagpal S, Jetty P (2017) Subclavian steal syndrome without subclavian stenosis. J Vascul Surg Cases Innov Tech 3: 129-131.
- Kim MS, Paeng JC, Kim KB, Hwang HY (2013) Left carotid-tosubclavian artery bypass grafting for recurrent angina caused by coronary-subclavian steal syndrome. Korean J Thorac Cardiovasc Surg 46: 84-87.
- Saha T, Naqvi SY, Ayah OA, McCormick D, Goldberg S (2017) Subclavian artery disease: diagnosis and therapy. Am J Med 130: 409-416.
- Westerband A, Rodriguez JA, Ramaiah VG, Diethrich EB (2003) Endovascular therapy in prevention and management of coronarysubclavian steal. J Vasc Surg 38: 699-704.
- Reis JMC, Kudo FA, Bastos MC, Sousa EMN, Oliveira MHB, et al. (2018) Endovascular therapy in coronary-subclavian steal syndrome: a clinical case. Int J Clin Med Res 5: 103-107.
- Kinno M, Niazi OT, Lorin JD, Chandrasekaran K (2017) The importance of subclavian angiography in the evaluation of chest pain: coronarysubclavian steal syndrome. Fed Pract 34: 26-30.
- Huibers A, Hendrikse J, Brown MM, Pegge SA, Arnold M, et al. (2017) Upper extremity blood pressure diference in patients undergoing carotid revascularisation. Eur J Vasc Endovasc Surg 53: 153-157.
- Cai XQ, Tian F, Zhou SS, Jing J, Hu W, et al. (2019) A rare case of non- ST-segment elevation myocardial infarction triggered by coronary subclavian steal syndrome. J Geriatr Cardiol 16: 378-380.
- Caesar-Peterson S, Qaja E (2019) Subclavian artery stenosis. In: StatPearls.
- De Roeck F, Tijskens M, Segers VFM (2019) Coronary-subclavian steal syndrome, an easily overlooked entity in interventional cardiology. Catheter Cardiovasc Interv 9: 1-6.
- Faggioli G, Pini R, Cremonesi A, Grattoni C, Longhi M, et al. (2014) Endovascular treatment of late coronary-subclavian steal syndrome. J Thorac Cardiovasc Surg 148: 2112-2116.
- Chen DW, Gao YH, Shi J, Yin YW, Zhang WQ (2020) Significance of hemodynamic assessment by pressure wire for endovascular therapy of subclavian steal syndrome. Interv Neuroradiol 26: 321-328.
- Sag S, Nas OF, Bedir O, Baran İ, Gullulu S, et al. (2016) Coronarysubclavian steal syndrome in a hemodialysis patient with ipsilateral subclavian artery occlusion and contralateral vertebral artery stenosis "Case Report". Anatol J Cardiol 16: 545-546.
- Che W, Dong H, Jiang X, Peng M, Zou Y, et al. (2017) Subclavian arftery sftenfing ffor coronary-subcflavfian sfteafl syndrome. Cardiovasc Interv 89: 601-608.
- Paty PS, Mehta M, Darling RC, Kreienberg PB, Chang BB, et al. (2003) Surgical treatment of coronary subclavian steal syndrome with carotid subclavian bypass. Ann Vasc Surg 17: 22-26.
- Galyfos GC, Kakisis I, Maltezos C, Geroulakos G (2019) Open versus endovascular treatment of subclavian artery atherosclerotic disease. J Vasc Surg 69: 269-279.
- Schillinger M, Haumer M, Schillinger S, Ahmadi R, Minar E (2001) Risk stratication for subclavian artery angioplasty: is there an increased rate of restenosis after stent implantation? J Endovasc Ther 8: 550-557.
- Miiller JC, Candemil PC, Loures JM, Zucco FM, Belz WE, et al. (2012) Coronary-subclavian theft syndrome: case report and literature review. Braz Vasc J 11: 166-170.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences