Decision Making Based on Clinicoanatomic Criteria to Select Open Versus Endovascular Repair for In nominate Artery Pseudoaneurysms Management
Raherinantenaina F, Andrianandraina MCG, Avotsihoarana TH, Rabemanantsoa R, Mampiadana ML, RakotoRatsimba HN and Rajaonanahary TMA
DOI10.36648/2634-7156.21.6.15
Raherinantenaina F1*, Andrianandraina MCG1, Avotsihoarana TH1, Rabemanantsoa R1, Mampiadana ML1, RakotoRatsimba HN2 and Rajaonanahary TMA1
1Vascular Surgery Department, CHU of Morafeno Toamasina, Madagascar
2General and Vascular Surgery Department, CHUJRA Antananarivo, Madagascar
- *Corresponding Author:
- Raherinantenaina F
Department of Vascular Surgery, CHU of Morafeno Toamasina, Madagascar
Tel: +261343852048
E-mail: heryfano1@gmail.com
Received Date: February 19, 2021; Accepted Date: March 24, 2021; Published Date: March 31, 2021
Citation: Raherinantenaina F, Andrianandraina MCG, Avotsihoarana TH, Rabemanantsoa R, Mampiadana ML, et al. (2021) Decision Making Based on Clinicoanatomic Criteria to Select Open Versus Endovascular Repair for In nominate Artery Pseudoaneurysms Management. J Vasc Endovasc Ther. 6 No. 3: 15.
Abstract
Background: The quality of present data on the effectiveness of endovascular repair (EVR) or open surgery (OS) to treat in nominate artery pseudoaneurysms (IAPs) is poor. The use of EV might simplify the procedure and provide a meaningful alternative for this condition, but it remains to be proved. We analyzed all IAP cases handled by OS or EVR from the literature to affect the decision-making for OS or EVR, basing the choice of intervention on clinicoanatomical data analysis.
Methods and results: All relevant literature reports of EVR and OS management of IAP were analyzed comparatively. Evidence-based data were extracted from the literature using Medline and Embase database resources. We totally recorded 73 cases, 50 from OS repair and 23 received EVR. Most patients who displayed hard signs or stable vital signs have been significantly managed by OS (94% vs. 57%) or EVR (43%vs. 8%) respectively. There was a significant predominance of iatrogenic injuries in the EVR group compared with those handled by OS (39%vs. 26%) except for patients who underwent endovascular stenting. OS was performed in the large majority of patients with severe blunt traumas (50%vs. 22%)(p=0.0328). Many patients classified as having cardiothoracic comorbidities benefited from EVR therapy (52%vs. 20%). Some cases in which arterial wounds were limited to the mid segment of the IA underwent EVR with significant proportion compared to the OS group (26%vs. 24%) (p=0.004). Patients in the EVR group had more operative failure and complications rates (39%) rather than those in the OS group (20%) (p< 0.05). Of the 23 patients managed by EVR, two had premature mortality (8.7%); of the remaining cases that received OS, the early mortality rate represented 6%. The significant causes for OS-related morbimortality were multiple organ failures, acute right heart insufficiency, pneumonia, deep vein thrombosis and hemorrhagic shock. Overall events for EVR-related death included stroke complications and massive hemorrhage from endoleak. The mid-term follow-up rate was significantly higher for the EVR group (57%) compared to the OS group (30%).
Conclusion: Compared with OS repair, EVR might represent an attractive alternative strategy for managing IAPs. The most primary option for OS included unstable patients, suffered from endovascular stenting, free of comorbidities and when the arterial wound is located at the distal portion of the IA.
Keywords
Endovascular repair; Innominate trunk; Brachiocephalic artery pseudoaneurysm; Open surgery revascularization
Introduction
False aneurysm, also known as pseudo aneurysm, occurs when a damaging force is applied to the arterial wall, allowing persistent extravasations of blood into the surrounding connective tissues. Innominate artery (IA) injuries are rare and accounted for roughly 0.4% of vascular wounds and 9% of chest penetrating vascular traumas [1]. In the past 30 years, less than a hundred cases of IA pseudo aneurysm (IAP) have been published since 1990. As detailed in Table 1, the vast majority of papers on this subject are case reports. Currently, open surgery (OS) remains the treatment of choice [2,3]. However, this surgical strategy possesses a considerable risk of operative morbidity and mortality. Adaptation of the endovascular repair (EVR) modality to supra-aortic vessels injuries has become popular in the recent decades [4]. EVR is a more practical technique than OS and effective as a lifesaving approach [1,4]. Awarding to our knowledge, no update with robust data has been previously established in this setting. The management continues unstandardized and there is no consensus concerning the optimal treatment, as to indications for EVR or OS techniques. For instance, the benefits of endovascular treatment, like short operative time and less trauma, shorter hospital and intensive-care unit stays, have typically been evaluated only with limited data on the basis of small samples [2,4]. Recently, a series study reported excellent results with mid-term follow-up (6-12 months), but only in a few young patients [4]. Accordingly, without a comparison of OS and EVR results in treating IAPs, it was premature to conclude that EVR technique is better than OS at dealing with these patients. We therefore conducted a systematic review of IAPs treated by EVR or OS repair in the literature to analyze comparatively characteristic differences between the both groups and identify valuable clinicoanatomical criteria for better decision-making of the therapeutic management.
| Authors /Year | Sex/age | Etiology | Delay Follow-up | Clinical presentation | Imagery Location | Treatment Outcomes |
|---|---|---|---|---|---|---|
| Lovelock et al 2020 [39] | M/36 | Blunt trauma Motor vehicle | Emergency 6 months | Polytrauma, shock, arm ischemia | CTA Proximal | MS, CPB, AI prosthetic graft |
| Pawar et al 2020 [33] | M/20 | Tracheostomy | 2 days 1 month | Severe bleeding, oozing (Peritracheostomy) | CTA+ART Mid | Failed primary repair Covered stent graft |
| Dapratiet al. 2020 | M/49 | Aortic surgery (Anastomotic) | 6 months 1 month | Mass | CTA AI junction | MS, CPB, aorto-carotid bypass |
| Volpe et al. 2019 [59] | M/62 | Blunt trauma | Emergency 24 months | Unstable patient Hemorrhage shock | CTA+ART Proximal | Covered stents (FA) Endoprothesis (DTA) |
| Safran et al. 2019 [17] | M/46 M/90 | Central vein catheterization Tunnel catheter (hemodialysis) | 3 hours 5 years 3 weeks 1 month | Chest and neck pain Shock, swelling Pulsatile neck | ART Proximal CTA+ART Proximal | Iliac leg extension stent grafts Stent grafts. BAA |
| Chen et al. 2019 [11] | F/63 | Aortic surgery (anastomotic) | 4 months 4 days | Asymptomatic | CTA Proximal | Covered stent AA + FA accesses |
| Choudhryet al. 2019 [9] | F/58 | Lung cancer (CRL), mycotic | 3 months 1 month | Bleeding (from chest wall wound), shock | CTA Proximal | Stent grafts + carotid-carotid bypass PTFE |
| Li et al. 2018 [52] | F/60 | Meniere’s disease | ----- 1 year | Pulsatile mass, dysphagia Dyspnea. Rupture | CTA+ART Distal | Kissing covered stent FA+BA access |
| Sibilleet al. 2017 [53] | F/60 | Blunt trauma Motor vehicle | 14 days 1 month | Chest pain | CTA, BAA Proximal | Stent graft + bypass |
| Choufaniet al. 2016 [58] | M/28 | Penetrating injury (shrapnel) | Emergency 1 month | Dyspnea Asymptomatic | CTA+ART Proximal | Covered stent FA open access |
| Terceros-A et al. 2016 [6] | M/43 | Blunt trauma | Emergency ------ | Multiple trauma Unstable patient | CTA Proximal | Conservative survey |
| Machado et al. 2016 | M/76 | Tracheostomy | 2 weeks 1 month | Bleed | CTA Proximal | Bifurcated bypass |
| Lee et al. 2015 [8] | M/55 | Anastomotic | 25 years 6 months | Chest pain | CTA Distal | Stent + PTFE |
| Rahimiet al. 2015 [48] | M/60 | Carotid surgery | ----- 10 months | Stroke, hemiparesis, arm ischemia | ART Mid | Covered stent |
| Roussel et al. 2015 [10] | F/42 | SVC stenting | 4 years 18 months | Hemoptysis | CTA+ART AIJ | Covered stent graft + Axilloaxillary bypass |
| Galanteet al. 2015 [13] | M/64 | Blalock-Taussig shunting | ----- 2 months | Headache, coma Stroke | CTA+ART Proximal | Stent graft Died (stroke) |
| Koorakiet al. 2015 [51] | F/74 | Hemodialysis catheter | 19 days 3 months | Hematoma, thrill, dysphagia, hoarseness | CTA+ART Mid | Covered stents FA access |
| Hodjatiet al. 2015 | F/29 | Blunt trauma | 1 month 3 months | Dyspnea, stridor, respiratory failure | CTA Proximal | Open repair, bypass Synthetic graft |
| Liu et al. 2014 [30] | M/45 | Blunt trauma | 61 hours 8 months | Back pain, dyspnea Respiratory distress | CTA, BAA Proximal | Quadrifurcated graft CPB, sternotomy |
| Boutayebet al. 2014 [38] | M/54 | Blunt trauma | Emergency 1 month | Multiple trauma | CTA Origin | MS, CPB Prosthetic graft |
| Brahmbhattet al. 2014 [44] | M/57 | Tracheostomy (Dilatation) | Emergency Weeks | Bleeding | CTA Mid | Open repair. Died |
| Azarconet al. 2014 [16] | M/25 | Blunt trauma Motorcycle | 2 days 5 days | Asymptomatic | CTA+AOT Distal | Covered stent-Viabahn, endoleack |
| Greene et al. 2014 [26] | F/3 | Explosion (metal fragment) | 6 days 1 week | Fragmentation injuries Hemothorax | CTA Distal | Manubriotomy, vein patch angioplasty |
| Fukuda et al. 2014 [54] | M/59 | Anastomotic | 2 weeks 8 months | Back pain | CTA+AORT Proximal | Covered stent BA access |
| Alagappanet al. 2014 | M/20 | Blunt trauma | 1 hour 1 month | Ischemic stroke Hemiparesis | CTA Proximal | Open surgery ETE anastomosis |
| Mosqueraet al. 2013 [40] | M/49 | Blunt trauma | 2 weeks 1 year | SVCS, respiratory distress Pulsatile swelling | CTA Distal | Pericardial patch CPB, sternotomy |
| Roan et al. 2013 [28] | M/76 | Infection | 2 weeks 5 years | Fever, sepsis | CTA Distal | MS, CPB, Extra-anatomic bypass |
| Philip et al. 2012 [42] | M/0.3 | Subclavian vein catheterization | 8 days 7 days | SVCS, respiratory failure | DUS+CTA Mid | Suture 7/0 Prolene |
| Durieuxet al. 2012 [43] | M/59 | Endocarditis Staphylococcus | 6 weeks 1 month | Mitral endocarditis | USD+MRA Proximal | Cryopreserved arterial homografts |
| Scantleburyet al. 2012 [56] | F/46 | Lymphoma surgery | 7 years 3 months | Painful pulsatile mass (anterior chest wall) | AORT Proximal | Covered stent FA access |
| Darkoet al. 2012 | M/23 | Blunt trauma Motor vehicle | Emergency 2 weeks | Neck and chest wall tenderness | CTA Proximal | MS, Bypass Dacron Aorto-innominate |
| Miliauskaset al. 2012 | M/58 | Chest traumas and surgery | 1 year 1 month | Dyspnea, massive hemoptysis | CTA+AORT Proximal | Aneurysmectomy |
| Takigushiet al. 2011 | M | Traumatic injury | ------ 1 month | ------- | CTA Mid | Open repair, CPB Artificial vessel |
| Caballero V et al. 2011 [5] | F/26 | Blunt trauma | 3 years | Mass, dyspnea | CTA | Non-mentioned |
| de Troiaet al. 2011 [45] | F/71 | Subclavian vein cannulation | Emergency 16 months | Acute chest pain Anemia | CTA Mid | Covered wall graft stent, FA access |
| Cordovaet al. 2011 [37] | M/32 | Blunt trauma | Emergency 30 days | Multiple trauma | CTA+ART Mid, BAA | AI Bypass (Dacron) + aortic stent graft |
| Mousaet al. 2010 [27] | M/51 | Blunt trauma Motor vehicle | 28 years 1 month | Cough, chest pain Acute dyspnea | X-Ray CTA | Sternotomy Dacron graft |
| Salcuniet al. 2009 | F/71 | Subclavian vein catheterization | 6 hours 16 months | Neck pain | CTA+ART | Wall stent |
| Kanwaret al. 2009 [47] | M/17 | Blunt chest trauma (football) | 3 months 2 years | Stroke (weakness) Pulsatile mass | CTA+ART Mid | Open repair |
| Ahmed et al. 2009 [49] | M/53 | Mediastinoscopy (biopsy) | 3 months 1 month | Asymptomatic | CTA+AORT Proximal | Covered stent grafts FA access |
| Wang et al. 2009 [62] | F/39 | Dilatational tracheostomy | 2 days 18 hours | Acute bleeding (tracheostomy) | ART Mid | Wallgraft stents Embolization. Died |
| Elahiet al. 2009 | M/79 | Coronary surgery | 10 years 1 month | Acute onset hoarseness Dysphagia | CTA (giant) Proximal | Bypass (Dacron) + Stenting graft |
| Guilbertet al. 2008 | F/79 | Jugular vein catheterization | 3 hours 1 month | Pulsatile blood return | ------ | Ministernotomy Suture |
| Rabuset al. 2008 [41] | F/27 | Blunt chest trauma | 4 years Weeks 1 month | Lung symptoms pneumonia | CTA+MRA Proximal | Sternotomy- bypass (Aorto-carotid-subclavian Dacron) |
| Rispoliet al. 2008 [35] | M/71 | Innominate artery stenting | 12 years 20 months | Chest pain, dyspnea Cough | CTA (giant) Proximal | Median sternotomy CPB, Bypass Dacron |
| Petrocheilouet al. 2008 [15] | F/65 | Hickman line insertion | 6 hours Death | Dyspnea, stridor, shock Instable patient | CTA Proximal | Median sternotomy Died (Hemorrhage) |
| Choi et al. 2008 [14] | M/49 | Blunt trauma | 14 months 1 month | Dyspnea, hoarseness | CTA (BAA) Proximal | Open surgery, CPB Prosthetic graft |
| Huang et al. 2008 [57] | M/36 | Blunt trauma Motor vehicle | Emergency 1 year | Severe chest back pain | CTA+ART Distal | Covered stent graft FA access |
| Vanhuyseet al. 2008 [29] | F/49 | Blunt trauma Clavicle luxation | ----- 1 month | Chest pain | CTA Mid | Open surgery Bypass |
| Ito et al. 2007 [18] | M/86 | Atherosclerotic ulcer | 4 months 1 month | Hemoptysis, hoarseness Neck pain | CTA+ART Mid | Large MS, Prosthetic graft (Dacron) |
| Augoustideset al. 2006 [34] | M/16 | Tracheostomy | 7 months 5 days | Hemoptysis | Giant IAP | Endovascular coiling FA, CPB |
| Sakamoto et al. 2006 [20] | M/64 | Endovascular stenting | 34 years 3 weeks | Dyspnea, cough, fever Repeated pneumonia | CTA+ART Mid | CPB, Prosthetic graft |
| Szetoet al. 2006 [46] | M/16 | Fistula (trachea and innominate) | 7 months 3 months | Bleeding, RDS Rupture | ART Proximal | Stent graft via carotid artery |
| Maddaliet al. 2006 [61] | F/50 | Jugular vein catheterization | 3 years 1 month | Pulsatile swelling, bruit | ART Distal | Thoracotomy, CPB Patch, Stent Gore-tex |
| Zoffoliet al. 2006 [60] | F/35 | Blunt trauma Vehicle crash | Emergency 1 year | Multiple trauma Respiratory insufficiency | CTA+AORT Distal | Laparotomy Stent graft via aorta |
| Tsutsumiet al. 2005 | M/62 | Innominate catheterization | 3 months 1 month | Intermittent chest pain | CTA+MRA Mid | Median sternotomy Prosthetic graft 8mm |
| Okubo et al. 2005 [31] | F/68 | Radiation Mediastinitis | 65 months 18 months | Chest skin ulceration Rupture | ART Proximal | Median sternotomy Bypass Dacron |
| Wells et al. 2005 [36] | M/20 | Blunt trauma | Emergency 6 days | Severe blunt injury | ART (BAA) Proximal | Median sternotomy Bypass grafting |
| Symbaset al. 2005 [3] | M/32 M/32 M/40 | Blunt trauma Blunt trauma Blunt trauma | Emergency 23 days Emergency 8 days Emergency 9 days | Multiple trauma, shock Multiple trauma Multiple trauma Chest and back pain | CTA+ART Proximal AORT AI junction ART Proximal | MS, AI PTFE graft interposition Failed EVAR MS, AI PTFE graft MS, AI PTFE graft interposition |
| Dhaliwalet al. 2005 [12] | M/20 | Blunt trauma | 5 months 3 weeks | RDS, SVCS, swelling Stridor | ART (giant) Distal | Thoracotomy + CPB Suture 4/0 |
| Reddiet al. 2005 [21] | M/46 | Stab injury wound | 26 years Months | Stridor | CTA + ART (giant) Mid | Large sternotomy Suture |
| Cothrenet al. 2005 [25] | M/35 | Blunt trauma Motor vehicle | Emergency 33 days | Severe chest injury | ART Proximal | Sternotomy Bypass PTFE 12 mm |
| Kaushalet al. 2005 | M/62 | Mycotic Staphylococcus | 4 months 6 weeks | Embolic stroke Hemiparesis, hoarseness | MRA Proximal | Median sternotomy CPB, Patch pericardia |
| Moise et al. 2004 [32] | F/46 | Blunt trauma Motor vehicle collision | Emergency 6 months | Multiple trauma Stable hemodynamics | CTA+AORT Proximal BAA | MS, CPB, graft interposition. |
| Bielbet al. 2003 | M/45 | Mediastinoscopy | ----- 1 month | ------ | Proximal | Failed Stent graft Aorto-carotid bypass |
| Walter et al. 2003 [19] | M/68 | Balloon angioplasty | 3 weeks 3 weeks | Recurrent laryngeal nerve palsy | MRA Distal | Open bypass + CPB Bifurcated Dacron |
| Amondet al. 2002 [7] | M/80 | Subclavian vein catheterization | ----- ----- | Thoracic pain | ------- | Thrombosed spontaneously |
| Pruitet al. 2002 [23] | F/74 | Mycotic stenting | 2 weeks Death | Distal septic emboli | CTA | Sternotomy + CPB Died |
| Axisaet al. 2000 [50] | M/21 | Blunt trauma Road accident | Emergency 18 months | Flail chest, hemothorax Severe injuries | CTA+AORT Mid | Cervicotomy Covered stent graft |
| Sommer et al. 2000 | M/19 | Blunt injury | 3 years 1 month | Stroke, hemiparesis | MRA+ART Distal | Surgical removal, saphenous vein graft |
| Chandler et al. 1999 [55] | M/20 | Blunt trauma Road accident | Emergency 1 month | Flail chest Hemopneumothorax | CTA + ART Proximal | Palmaz stent via FA and CCA |
| Harvey et al. 1999 | M | Penetrating injury (gunshot) | 2 months 1 month | Bilateral hemiparesis Hemiplegia | ART Distal | Carotid-brachial bypass |
| Ihayalet al. 1999 | M/69 | Subclavian vein catheterization | 7 months 1 month | Hoarseness | CTA + ART | Sternotomy Patch Gore-Tex |
| Boulahyaet al. 1999 | M/33 | Jugular vein catheterization | 1 year 23 months | Hemoptysis, swelling | USD + CTA + ART | Cervicosternotomy Shunt, patch Dacron |
| Yamashiroet al. 1998 | F/28 | Trauma traffic accident | Emergency 1 month | Blunt chest trauma | AORT Proximal | Open surgery Bifurcated Dacron |
| Ruebbenet al. 1997 | M | Polytrauma | Emergency 1 month | Polytraumatized patient | Trunk Mid | Open surgery Stent graft placement |
| Lobo et al. 1997 | M/17 | Penetrating injury (rifle) | 3 hours 1 year | Hemoptysis | CTA+AORT Proximal | Extended MS, Dacron graft |
| Souryet al. 1995 | F/63 | Subclavian vein catheterization | 2 days 1 month | Asymptomatic | CTA + ART Mid | Open surgery Suture |
| Maria et al. 1995 | M/25 | Blunt chest trauma | Emergency 18 months | Resuscitated patient (cardiac arrest) | CTA Proximal | MS, AI bypass (PTFE) |
| Kraus et al. 1993 | F/40 | Blunt chest trauma | Emergency 14 months | Multiple trauma Cutaneous wound | CTA+ART Mid | SCI+MS ETE anastomosis |
| Folliset al. 1992 | F/55 | Subclavian vein catheterization | 2 months 1 month | Dyspnea | CTA + ART | Sternotomy + CPB ETE anastomosis |
Abbreviations: AA: Axillary Artery; AIA: Aorto-innominate Artery; AIJ: Aorto-innominate Junction; AORT: Aortography; ART: Arteriography; BA: Brachial Artery; BAA: Bovine Aortic Arch; CCA: Common Carotid Artery; CPB: Cardiopulmonary Bypass; CTA: Computed Tomography Angiography; DTA: Descending Thoracic Aorta; DUS: Doppler Ultrasound; ETE: End-to-End; FA: Femoral Artery; MS: Median Sternotomy; PO: Postoperative; PTFE: Polytetrafluoroethylene; RDS: Respiratory Distress Syndrome; SCI: Supra-Clavicular Incision; SVCS: Superior Vena Cava Syndrome
Table 1 Baseline data from published case reports regarding brachiocephalic artery pseudo aneurysms management (n=84).
Methods
Study Identification
Articles published between 1990 and May 2020 in the MEDLINE and EMBASE databases were searched online. The descriptors used to find titles of possible interest were «post-traumatic innominate artery pseudo aneurysm», «surgical and endovascular brachiocephalic artery repair», «supra-aortic vessels injury», «covered stent and in nominate artery pseudo aneurysm». After reading the abstracts online, 81 articles were downloaded for complete reading. All papers referenced were read selectively, and the review finally included 78 articles (81 cases). These selected reports covered a period of 30 years, while 14 (17.3%) were published from 1992 to 2000, 32 (39.5%) from 2001 to 2009, and 37 (45.7%) between 2010 and May 2020.
Criteria for inclusion and data extraction
We included relevant papers with comprehensive information on the following criteria: patient demographics including age, gender; etiology and mechanism of vascular trauma like catheterization and surgery, blunt trauma, inflammatory or infectious disease and penetrating injury; most comorbidities; clinical presentations and symptoms; concurrent injuries; radiological findings and details of treatment; arterial wound characteristics; perioperative complications and mortality; follow-up and outcomes. Abstracts and case series without specified information according to the stated selection criteria were excluded. All data were extracted from the article texts.
Statistical analysis
Patient data were collected in a Microsoft Excel database. The exploratory data analyses checked the distribution of values and presented the results as the mean and standard variation for numerical variables. Nominal data were presented in the form of frequencies and associated percentages. Differences between patients receiving OS and EVR were checked using the chi-squared test or Fisher’s exact test for categorical variables. All statistical tests were tailed, and p<0.05 was considered statistically significant. All statistical analyses were performed with Epi Info 7TM software, version 7.2.2.6.
Results
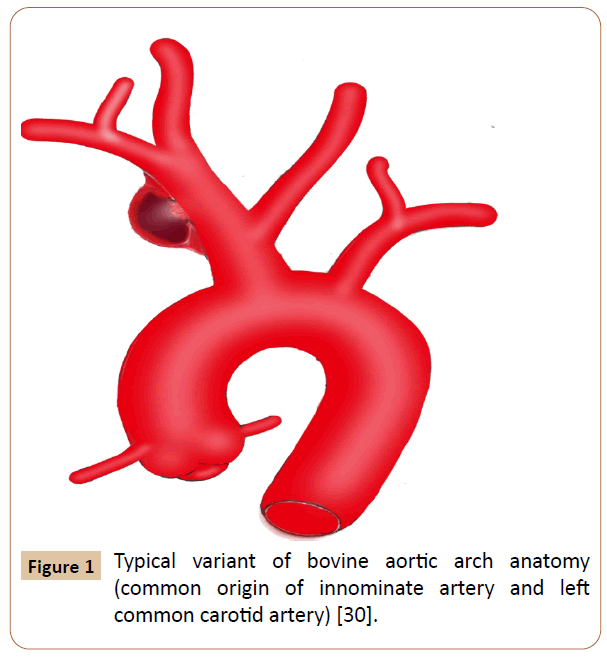
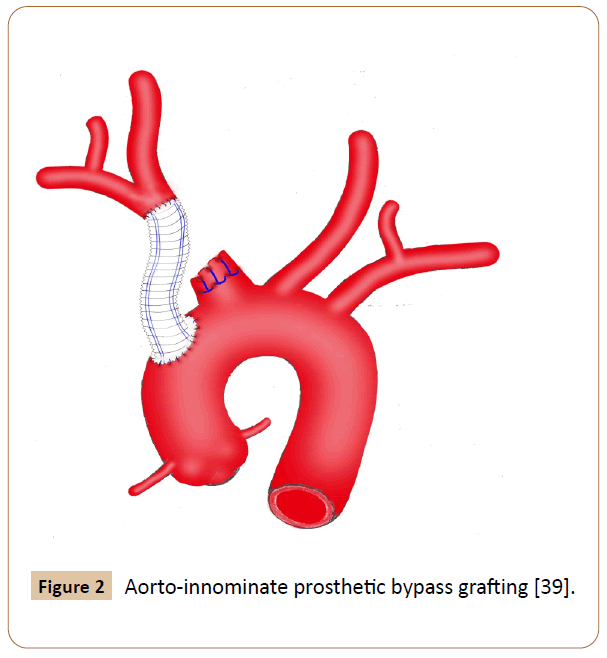
Eighty four cases were recorded. Eight patients receiving hybrid techniques were reviewed separately because we have not identified any significant difference for this group compared to the OS or EVR group. Among them, 50% were male, 87.5% had comorbidities and hard signs, 62.5% suffered from iatrogenic injuries, 25% presented with bovine arch anatomy (Figure 1), 75% underwent endovascular stenting and prosthetic bypass grafting concomitantly with minor complications in 50% of cases, no deaths occurred. Additionally, we excluded three cases, two of which seems to support no specific management and the other recovered without any surgical intervention [5-7]. The latter was observed in an elderly patient who complained of chest pain after percutaneous subclavian vein catheterizations, in which the pseudo aneurysm has thrombosed spontaneously [7]. Overall baseline clinical characteristics of the 73 patients evaluated are indicated in Table 2. The study included 52 (71%) men and 21 (29%) women with a mean age of 46 ± 21 years (ranges 3 months-90 years). Fifty patients underwent OS (68.5%) and 23 received EVR (31.5%). The primary OS techniques used included graft interposition or prosthetic bypass (70%) (Figure 2), lateral sutures (14%) patch angioplasty (10%) and end-to-end anastomosis (6%). The two groups were approximately similar in age, gender and diagnostic delay. Sixty patients (82.2%) presented with hard signs. Of these, 47 cases received OS procedures with significant predominance (94% vs. 57%) especially for patients whose evident symptoms of stroke or infections were present. Some patients were asymptomatic or displayed stable vital signs (19%). The overwhelming majority of them were managed endovascularly (44%vs. 8%) (p=0.0104). Regardless of the injury mechanism, iatrogenic (30%) and blunt (41%) traumas attended the most prominent events of all etiologies. Notably, many iatrogenic injuries were repaired by EVR (39%vs. 26%) with p< 0.05. Blunt traumas were significantly more frequent in the OS group (50%vs. 22%). OS repair was performed in the large majority of patients with penetrating injury (18%vs. 9%) (p=0.003). Most comorbid patients (30%) benefited from EVR procedure (52%vs. 20%) with p<0.001. Other perioperative data and outcomes are outlined in Table 3. Concurrent injuries were present in 34 patients (47%) with no significant difference between the two groups (48%vs. 46%). Giving to the radiologic findings obtained from CTA, arteriography and operative views, OS had handled many patients who developed loco regional complications without statistical significance (60%vs. 48%) especially for those experienced giant pseudo aneurysms. There were more injuries with contained rupture and thromboembolism events in EVR (30%vs. 22%) and OS (12%vs. 9%) groups respectively (p<0.02). Several patients in whom arterial tears were limited to the mid segment of the IA benefited from EVR procedure with significant proportion compared with those underwent OS repair (26% vs. 24%). Injuries to the distal portion of the IA have been typically managed by OS (16%vs. 13%) (p<0.05). In the OS group, the cardiopulmonary bypass (CPB) using was significantly more frequent in the management of proximal injuries (18%vs. 8%). The length of arterial tears was available in 35 patients (48%), in which no significant difference was discovered between the two groups (54%vs. 35%). A bovine arch anatomy was encountered in nearly 7% of patients, of which all arterial tears were located within the proximal IA segment, and most of them underwent OS repair (80%). Patients in the EVR group had more operative failure and complications rate (39%) rather than those in the OS group (20%) (p<0.002). The early reoperation rate was also significantly higher in the EVR group (13%vs. 4%). The length of hospital stay was stated in 18 patients (25%), which was longer in the OS group compared with the EVR group (p<0.007). The mean follow-up was 5 months in the OS group versus 9 months in the EVR group (p=0.5285). The mid-term follow-up of EVR was significantly higher than those of OS (57%vs. 30%). There were 5 mortalities totally (7%), including 4 early within 30 days after operations and 1 late over 30 days following procedures. The mortality rate was higher in the EVR procedure (9%) compared with the OS (6%) (p<0.05). The 3 early mortalities of OS were caused by multiple organ failures, acute right heart insufficiency and hemorrhagic shock. The other early death occurred from a massive bleeding as a result of failed EVR processes. A late mortality was observed in an EVR patient who died from stroke complications. The main EVR-related morbidity consisted of early reintervention, endovascular stenting and coil embolization failures, type-II endoleak, and pneumonia and stent restenosis.
| Variables | Open repair (50) | Endovascular repair (23) | Hybrid repair (8) | p value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Mean age | 45.6 ± 20.9 | 46.7 ± 21.5 | 53.7±14.8 | 0.084395 |
| Gender | ||||
| Male | 37(74) | 15(65.22) | 4(50) | NS |
| Female | 13(26) | 8(34.78) | 4(50) | 0.000993 |
| Diagnostic delay (month) | ||||
| < 1 | 28(56) | 14(60.87) | 2(25) | 0.009237 |
| [1-12] | 13(26) | 4(17.39) | 1(12.5) | 0.056948 |
| > 12 | 8(16) | 1(4.35) | 4(50) | 0.176482 |
| Imprecise | 1(2) | 4(17.39) | 1(12.5) | 0.025492 |
| Hard signs | 47(94) | 13(56.52) | 7(87.5) | 0.000177 |
| Superior vena cava syndrome, respiratory distress syndrome, dyspnea, stridor | 10(20) | 2(8.7) | 2(25) | 0,099655 |
| Pulsatile bleeding, hemoptysis, pulsatile hematoma (chest, neck), pulsatile mass, thrill, audible bruit, shock, instable patient, polytrauma, severe injury, multiple trauma, neck and chest tenderness (enlarging hematoma) | 24(48) | 9(39.13) | 5(62.5) | NS |
| Stroke (hemiparesis, headache, coma), arm ischemia, nerve palsy | 7(14) | 2(8.7) | --------- | 0.024815 |
| Endocarditis, pneumonia, mediastinitis, septic emboli, sepsis | 6(12) | -------- | --------- | |
| Soft signs | 4(8) | 10(43.49) | 1(12.5) | 0.010421 |
| Asymptomatic patient, stabilized patient Isolated pain (chest, neck, back) Stable hematoma or non-pulsatile swelling, anemia Hoarseness, dysphagia | ||||
| Etiology of the pseudoaneurysm | ||||
| Iatrogenic injury Open surgery Vascular catheterization Endovascular stenting Medical intervention (tracheostomy, mediastinoscopy) | 13(26) 2(4) 7(14) 4(8) 2(4) | 11(39.13) 4(17.39) 5(21.74) ---------- 4(17.39) | 5(62.5) 2 (25) 2(25) --------- 1(12.5) | 0.000182 0.000438 0.000005 0.000438 |
| Blunt trauma Penetrating injury Inflammatory disease Infection | 25(50) 5(10) ------ 3(6) | 5(21.74) 1(4.35) 1(4.35) ---------- | 2(25) --------- --------- 1(12.5) | 0,032873 0.055547 0.219944 |
| Atherosclerotic ulcer (spontaneous perforation) | 1(2) | ---------- | ---------- | |
| Comorbidities | 10(20) | 12(52.17) | 7 (87.5) | 0.000001 |
| Tetralogy of Fallot | -------- | 1(4.35) | ---------- | |
| Coronary artery disease, ischemic cardiomyopathy, diabetes mellitus, dilative cardiomyopathy | 2(4) | ---------- | ---------- | |
| Aortic arch replacement, aortic regurgitation/dissection | 1(2) | 1(4.35) | 1(12.5) | |
| Chronic obstructive pulmonary disease Coronary heart disease, coronary surgery, left cardiac failure, chronic renal failure | ------ ------ | 1(4.35) --------- | 1(12.5) | |
| Mediastinal lymphoma/carcinoma, chemoradiation Tracheostomy, thymoma, sternotomy | 1(2) 1(2) | 2(8.7) --------- | 1(12.5) 2(25) | |
| Lung carcinoma, chemoradiation, lobectomy | 1(2) | --------- | 1(12.5) | |
| Cerebral palsy, cerebral tumor (meningioma) | ----- | 2(8.7) | --------- | |
| End stage renal insufficiency, hemodialysis | 1(2) | 1 (4.35) | --------- | |
| Meniere’s disease | ----- | 1(4.35) | --------- | |
| Type-2 diabetes, heavy smoking, multi-drug treated hypertension | 2(4) | 1(4.35) | 1(12.5) | |
| Laryngeal cancer, laryngectomy, chemoradiotherapy | 1(2) | 1(4.35) | --------- | |
| Hemicolectomy (adenocarcinoma), chemotherapy | ----- | 1(4.35) | --------- |
Table 2 Baseline clinical characteristics in the 81 cases evaluated.
| Variables | Open repair (50) | Endovascular repair (23) | Hybrid repair (8) | p value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Radiologic data | ||||
| CTA | 20(40) | ---- | ---- | |
| CTA + Arteriography | 14(28) | 17(73.91) | 7 (87.5) | 0.000052 |
| MRA | 6(12) | ---- | ---- | |
| Arteriography | 9(18) | 6(26.09) | 1(12.5) | 0.000052 |
| Concurrent injuries | 23(46) | 11(47.83) | 4(50) | NS |
| Left common carotid artery occlusion | 2(4) | ---- | ||
| Superior vena cava fistula, superior vena cava compression | 2(4) | 1(4.35) | ---- | |
| Airway obstruction, tracheal compression, tracheal deviation | 4 (8) | ---- | 1(12.5) | |
| Tracheal obstruction, repeated pneumonia, tracheal fistula | 6(12) | 3(13.04) | 1(12.5) | |
| Common carotid artery–internal jugular vein fistula | ------ | 1(4.35) | ----- | |
| Common carotid artery laceration/thromboembolism | 3(6) | ---- | ----- | |
| Innominate artery dissection/transection/obstruction | 2(4) | 2(8.7) | ----- | |
| Subarachnoid hemorrhage, spinal cord contusion, paralysis | ------ | 1(4.35) | ----- | |
| Aortic dissection, aorto-innominate junction injury | 2(4) | 2(8.7) | 2(25) | |
| Descending thoracic aorta transection/cardiac injury | 2(4) | 1(4.35) | ||
| Locoregional complications of the pseudoaneurysm | 30(60) | 11(47.83) | 2(25) | NS |
| Giant pseudoaneurysm (diameter ≥ 6 cm) Contained rupture Thrombosis/embolism | 13(26) 11(22) 6(12) | 2(8.7) 7(30.43) 2(8.7) | 2(25) ------ ------ | 0.211608 0.000263 0.011076 |
| Arterial wound localization | ||||
| Proximal portion | 25(50) | 11(47.83) | 4(50) | NS |
| Mid portion | 12(24) | 6(26.09) | 2(25) | 0.004623 |
| Distal portion | 8(16) | 3(13.04) | 2(25) | 0.009237 |
| Imprecise | 5(10) | 3(13.04) | ------ | 0.000065 |
| Arterial tear length | ||||
| ≤ 10 mm | 17(34) | 6(26.09) | 1(12.5) | 0.098553 |
| >10 mm | 10(20) | 2(8.7) | ------ | 0.099655 |
| Bovine-type aortic arch anatomy | 4(8) | 1(4.35) | 2(25) | 0.025492 |
| Cardiopulmonary bypass use | 16 (32) | 1(4.35) | 1(12.5) | 0.520998 |
| Perioperative complications | 8(16) | 6(26.9) | 4(50) | 0.000002 |
| Pneumonia, deep vein thrombosis, sepsis, respiratory failure | 3(6) | 1(4.35) | ------- | |
| Bleeding and massive amounts blood transfusion | 2(4) | ----- | 1(12.5) | |
| Type-II endoleak blush | ----- | 2(8.7) | 1(12.5) | |
| Endovascular stenting or coil embolization failure | ----- | 2(8.7) | 1(12.5) | |
| Hemorrhage, cardiac arrest, acute renal insufficiency | 3(6) | 1 (4.35) | ------- | |
| Fistula between trachea and innominate artery | ------ | ----- | 1(12.5) | |
| Reoperation | 2(4) | 3(13.04) | 1(12.5) | 0.000005 |
| Stent restenosis from intimal hyperplasia (2 months) Endoleak (18-hour, 4-day, 19-day) | ---- ---- | 1(4.35) 2(8.7) | 1(12.5) | |
| Unstable sternum (6 days), failed primary repair (bleeding) | 2(4) | ----- | ------- | |
| Perioperative death | 3(6) | 2(8.7) | ------- | 0.000005 |
| Stroke complications | ---- | 1(4.35) | ||
| Multi-organ failure | 1(2) | ----- | ||
| Acute right heart failure | 1(2) | ----- | ||
| Hemorrhagic shock and electromechanical dissociation | 1(2) | 1(4.35) | ||
| Hospital stay length(days) < 10 ≥ 10 | 6(12) 7(14) | 5(21.74) ----- | ------- 1(12.5) | NS |
| Follow-up (month) | ||||
| Short-term < 2 | 31(62) | 9(39.13) | 4(50) | NS |
| Mid-term [2-24] | 15(30) | 13(56.52) | 4(50) | 0.000001 |
| Long-term (5-year) | 1(2) | 1(4.35) | ------- | NS |
CTA: Computed Tomography Angiography; NS: Non-Significant; MRA: Magnetic Resonance Angiography.
Table 3 Other perioperative data and outcomes.
Figure 1 Typical variant of bovine aortic arch anatomy (common origin of innominate artery and left common carotid artery) [30].
Figure 2 Aorto-innominate prosthetic bypass grafting [39].
Discussion
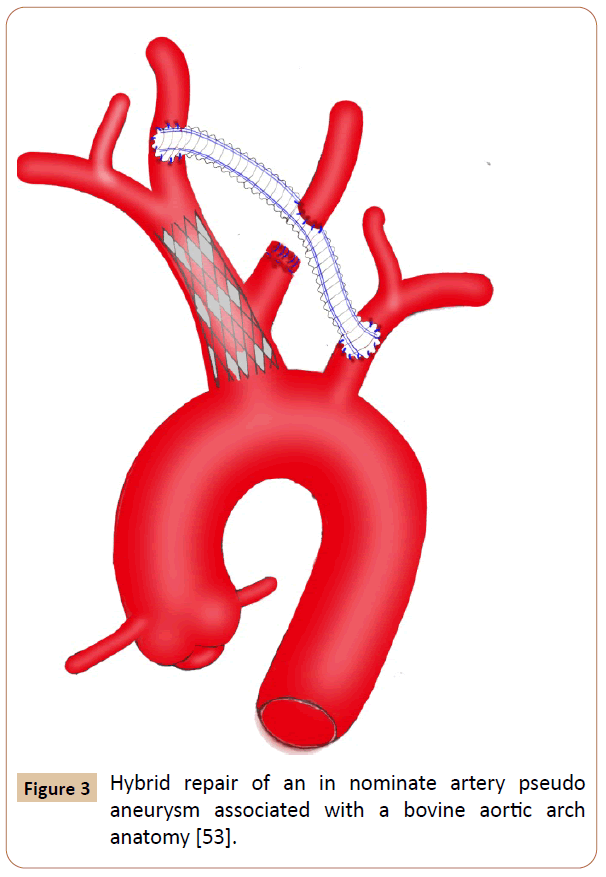
This review documented that OS and EVR techniques maintained their critical potential purpose and usefulness, as both were advantageously effective and complementary. Hybrid techniques combining extra-anatomic cervical bypass and endovascular stenting have been successfully used in both symptomatic and comorbid patients [8-10]. The general objective of these repair methods is to avoid thromboembolism, bleeding from rupture and nerve compression from mass effects secondary to massive hematoma. The specific indications were to alleviate compressive symptoms of surrounding vital structures such as trachea, esophagus and superior vena cava (SVC) [15-17]. The decision whether to treat with open, endovascular, or both measures should be guided by surgeon experience and the patient’s overall symptomatology and comorbid conditions. The utmost criteria should include aortic arch anatomy patterns (bovine aortic arch) and other supra-aortic vessels involvement, location of the arterial wound, and diameter and length of the IA. Granting to the outcomes as mentioned above, OS was reported to be morbid and fatal procedure in some patients. The reason is mostly resulted from the surgical technique itself requiring aggressive median sternotomy, from which extension into the right cervical region is generally [3,18]. This technique has already been associated with sternum instability resulting in an early reoperation [19]. Sometimes, when the CPB is unavailable, other accesses such as concomitant cervicotomy or supra-clavicular incisions and anterolateral thoracotomy have been needed to get better the operative view and to upgrade the quality of control procedures intraoperatively [2,12,20-26]. A maximal vascular exposure also required dislocation of the sternoclavicular joint and resection of the proximal portion of clavicle [27]. Markedly, all patients with thoracic surgery antecedents may be exposed to hazardous events due to vascular dissection and control gesture, which became difficult and hemorrhagic because of multiple fibrous adherences as a result of inflammatory surrounding structures and chronic hematoma [9,18,21,23,28]. The operative length may also be longer particularly in the bifurcated or quadrifurcated prosthetic bypass carrying out (215 ̶ 224 min) [24,29-32]. Rarely performed, lateral suture and end-to-end anastomosis techniques are swift but unsafe in the large arterial substance loss length (> 10 mm), which is frequently associated with high risk of tension, stenosis and stitches dehiscence [33]. Aortic clamping also figures among the incursive patterns of the OS approach. To reduce the operative risks, some investigators employed protective measures for cerebral perfusions, like passive aorto-carotid shunting, electroencephalogram monitoring and CPB using with deep hypothermia and circulatory arrest for management of associated aortic arch injury, large pseudo aneurysms, bovine aortic arch anatomy, and proximal and complex injuries [12,14,18-20,23,28,30-39]. The purpose of the CPB, further, facilitated the subclavian and carotid arteries control with rupturing the pseudo aneurysm [12,40]. Even so, any intempestive maneuver leading to excessive handling of the pseudo aneurysm must be avoided before making a distal clamping to prevent the cerebroembolic risk [24]. Despite the disadvantages of the OS, several situations and patient characteristics benefited from it, as the EVR failed or unavailable [3]. Kieffer et al reported their experience with the most previous cardiovascular OS techniques of 27 IA aneurysms over a 7-years interval. Overall operative morbidity (22%) and mortality (4.3%) rates appear acceptable, and no recurrence or infectious complications has been reported [24]. Also, du Toit et al had good or excellent results in 34 patients who suffered from penetrating IA injuries and treated by OS repair [2]. There were 79% and 6% of survival and operative complications rates respectively. The successfulness of the OS may be explained by achievement of manifold repairs working side- or cross-clamping technique without CPB [2-4,9,10,21,24-26,36,37,41,42]. Nevertheless, cross-clamping the IA with no cerebral perfusion assistance is not recommended in elderly populations because they are likely to have insufficient cerebral artery communications [18]. In addition, pediatric populations and absence of adequate landing zones represent an argument for OS therapy [8,20,26,27,30,37,41,42]. The most primary option for this surgery should include hemodynamically unstable patients, iatrogenic injuries resulting particularly from iterative endovascular maneuvers, free of major comorbidities, youthful populations, mycotic pseudo aneurysms, presence of bovine aortic arch, and injuries to the mid or distal portion of the IA [3,4,14,18,23,28,30,32,35,37-39,43]. In this group, perioperative hypotension, respiratory complications, systemic infection, serious bleeding, and anemia were all linked to the postoperative cardiac morbimortality [2,15,23,24,44]. Hazardous management of a bovine trunk and emergency surgery for a ruptured aneurysm were also reported as a fatal condition with high postoperative mortality rate (> 50%) [2,24]. The top two factors of significant mortality included coma at presentation and associated injuries [2]. Generally foreseeable, intraoperative exsanguinations, irreversible hypovolemic shock, cardiac tamponade, respiratory difficulties necessitating artificial ventilation, extensive cerebral infarction, neurologic deficit and multiple organ failures accounted for the principal perioperative complications reported [2,3,15,23,24,33,45,46]. Vascular complications and large hemorrhage volume are associated with prolonged hospital stay and increased morbidity risks [3]. The most common reported large hospital stay length ranged from 5 to 35 days [3,20,25,26,30,35,37,39,41,42]. Although there is insufficient evidence for follow-up, the mid-term results were satisfactory in terms of survival and free-relapse [2- 4,21,30,31,32,35,39,40,47]. Otherwise, surgical repair is so fraught with complications, and despite continued advances in medicine and critical care, morbidity and mortality events may occur. Such constraint is presumably at the origin of motive way and reason to support EVR techniques [4]. Currently, several authors have reported the results of EVR for the management of IAP, and it has subsequently been accepted widely as a therapy option [2,4,15,16,17,33,34,45,48-58]. Adaptation of EVR principles is similar to those employed in the management of supra-aortic vessels injuries, and successful intervention has been advocated. This repair is minimally invasive and faster than OS; however, it is crucially dependent on several criteria including the likelihood of stent infection, and the surgeon’s endovascular experience [4,17,33]. Many diagnostic conditions were handled by EVR techniques in patients with complex anatomy setting and history of multiple surgical procedures in the mediastinum, and in the presence of concomitant injuries and mediastinal carcinomas [33,37,46,49,53]. The remaining indications should include incomplete vascular rupture or transection confirmed by angiography, and compatible anatomy with stable landing zones which were commonly achieved within the proximal and mid portions of the IA [2,4,9,10,17,20,38,57]. Another basic option for EVR approach has been suggested to treat large or ruptured aneurysms with erosion of the sternum that was a contraindication to sternotomy because of the potential catastrophic hemorrhage risk [10,13,24,56]. With the technical difficulty and resulting danger of OS approaches, EVR offers an appealing solution, as it allows rapid control of hemorrhage with minimal physiologic impact on the patient [1,4,9,10,17,33,59]. In emergency situations, the decision-making could be performed in stabilized patients and in cases of high surgical risks, for which an eventual OS repair should be considered afterward [3,9,10]. To prevent a delicate dissection, a few studies have reported successful management with covered stent-grafts, wall-graft endoprosthesis, balloon-expandable covered stent, Palmaz stent, Amplatzer device, iliac leg extension stent grafts, bare stent, and kissing stent-grafts [2,4,10,15,16,17,33,34,45,48-60]. The fully percutaneous apical access is often associated with lower blood loss and a shorter hospitalization (1−8 days) in appropriately selected patients [4,15,48,56,59] compared with the surgical approach requiring sternotomy or thoracotomy (5−45 days) [2,3, 12,19,20,25,26,28,37,39,41]. Beforehand, overstenting of common carotid artery (CCA) origin has been unsafe in more distal lesions [2,20]. Currently, this was successfully challenged by the report of Li et al, which described the use of kissing stentgrafts for the treatment of focal lesions within the IA bifurcation [52]. In the other extremity, percutaneous device closure of pseudo aneurysm arising from the junction of the IA and the aorta might now be able to be performed safely [56]. The effectiveness of EVR is also associated with distinguished technical success and possibility to treat concurrent injuries such as arteriovenous or tracheo-innominate fistula, IA obstruction and transection of the descending thoracic aorta (DTA) [2,13,33,37,59]. In some cases, EVR procedures may be facilitated by exposure of the arterial access in advance, especially for CCA, and brachial and femoral arteries [33,34,46,50,58]. At intervals, EVR management could be achieved via aorta during laparotomy for abdominal damage control [60]. Whatever the surgeon’s ability, this technique is typically exposed to endoleak risk as well as isolated coil embolization failure that was already unable to ensure any IAP exclusion [3,9,34,61,62]. At mid-term follow-up, the pseudoaneurysm exclusion is consistently observed without a significant incidence of endoleak or conversion to open repair [2,4]. As aforementioned, EVR can be reserved as a bridge to open repair once patients have stabilized and local infection control is established [3,9]. It is, in addition, an acceptable treatment modality for patients with a limited life expectancy who would benefit from a short hospital stay and earlier discharge and will be unexposed to the long-term risks. EVR, however, may be complicated by enlargement of the arterial tear leading to a patent sac, requiring subsequently coil embolization and coverage for successful exclusion [9,46,56,61]. The present review supported as well the latest relative contraindication to the EVR therapy for managing IAPs [2]. In fact, injuries in close proximity to the aortic arch or innominate bifurcation should be managed by extra-anatomic crossover bypass with carotid-subclavian transposition and stent graft placement [2,9,10]. EVR was also not compatible with arterial tear length superior or equal to the diameter of the IA, and in the patient diagnosed as having a bovine aortic arch with life-threatening airway compression and severe hemorrhage [30,32,37,61,62]. Actually, the management of the bovine aortic arch trends to be achieved consistently by EVR or hybrid fashion (Figure 3) even in the presence of hemorrhagic shock and transection of the DTA [17,53,59]. Potential concerns with stent-graft placement include stenosis within the stent, despite the antiplatelet therapy, and the possibility of requiring lifelong surveillance, particularly in the trauma setting, in which the patients tend to be young and often not compliant [23,57,58]. At last, vascular access site complication is one of the important and most frequent causes of morbidity following EVR but it was undescribed in this review. Earlier reports on EVR have disseminated promising results; however, only a few case reports and limited series with restricted follow-up were published. Some authors documented excellent results with long-term patency (70-98%), but only in patients who underwent percutaneous transluminal angioplasty for atherosclerotic stenoses and occlusions [63,64]. The crucial question of durability, therefore, remains unanswered. Whatever, it is evident that the presence of a stent graft could complicate a later surgical repair in many ways, and primary OS is preferable for patients considered eligible for this therapy [23,26]. Endovascular treatment positively represents a therapeutic alternative, particularly in emergency and in polytraumatized patients [4,33]. Nevertheless, this process was associated with higher technical failure and morbimortality rates compared with those observed in the OS repair. Therefore, surgical repair continues the preferred treatment because of the lack of long-term data [3,39,65].
Figure 3 Hybrid repair of an in nominate artery pseudo aneurysm associated with a bovine aortic arch anatomy [53].
Limitation
Some useful information details were unavailable in old data and images articles such as operative technique, hospital stay length, and follow-up.
Conclusion
This review showed that IAPs management can be achieved safely and effectively by EVR or OS regarding 30-day and mid-term morbimortality rates. Hemodynamically stable patients, iatrogenic injuries, antecedent of comorbidities, contained rupture and wound located at the middle portion of the IA were suitable for EVR procedure. OS repair may be proposed in young patients, displayed hard signs, suffered from endovascular stenting and blunt trauma, free of cardiothoracic comorbidities, diagnosed with a bovine aortic arch anatomy, and when the arterial tear is located at the distal segment of the IA. Proximal traumatic injuries are also best treated with a bypass from the ascending aorta to the involved IA, especially when not anatomically suitable for EVR. Hybrid repair with an extra-anatomic cervical bypass may be an ideal fashion for complex injuries or in high-risk patients [8-10,53,61].
Conflicts of Interests
We stated no conflicts of interests and funding on this article.
References
- Dua A, Desai SS, Holcomb JB, Burgess AR, Freischlag JA (2014) Clinical review of vascular trauma. Springer: Berlin.
- Du Toit DF, Odendaal W, Lambrechts A, Warren BL (2008) Surgical and endovascular management of penetrating innominate artery injuries. Eur J Vasc Endovasc Surg 36: 56-62.
- Symbas JD, Halkos ME, Symbas PN (2005) Rupture of the innominate artery from blunt trauma: current options for management. J Card Surg 20:455-459.
- Jia W, Liu J, Li J, Tian X, Jiang P, et al. (2020) Treatment strategy for traumatic innominate arterial injury. Chinese J Traumatol 23: 10-14.
- Caballero-Vázquez A, Fernández-Vázquez E, Ruiz-Carazo E (2011) Post-traumatic pseudo aneurysm of the in nominate artery: a rare presentation of tracheal stenosis. Arch Bronconeumol 47:613-616.
- Terceros-Almanza LJ, Barea-Mendoza J, Chico-Fernández M, García-Fuentes C, Navarro-Cutillas V, et al. (2016) Post-trauma pseudo aneurysm of the proximal innominate artery. Med Intensiva 40:186-195.
- Amond L , Haxhe JP, Frankart L, Denef R, Liessenborghs L (2002) Spontaneous thrombosis of an iatrogenic pseudoaneurysm of the arterial brachiocephalic trunk following central venous catheterization. Ann Fr Anesth Reanim 21:530-533.
- Lee CW, Song S, Choi SU, Kim SH, Lee HC (2015) Hybrid repair for anastomotic pseudo aneurysm on the in nominate artery following blunt chest trauma. J Card Surg 30:836-838.
- Choudhry AJ, Shaw P, Gonzalez L, Costanza MJ (2019) Hybrid endovascular exclusion of a bleeding innominate artery pseudoaneurysm in a patient with no open surgical options. J Vasc Surg Cases Innov Tech 5:132-135.
- Roussel A, Fabre D,Fadel E, Angel C, Dartevelle P (2015) Hybrid treatment of an aortic pseudo aneurysm arising at the innominate artery junction secondary to superior vena cava stenting. J Vasc Surg Cases 1:127-129.
- Chen RC, Kwon M, Levi D, Moriarty JM (2019) Use of a covered stent to exclude an aortic-brachiocephalic conduit pseudoaneurysm. Tex Heart Inst J 46:143-146.
- Dhaliwal RS, Luthra S, Goyal S, Behra S, Krishna R, et al. (2005) Traumatic giant pseudo aneurysm of in nominate artery. Asian Cardiovasc Thorac Ann 13:369-371.
- Galante O, Grinberg G, Sandro G, Almog Y (2015) Brachiocephalic pseudo aneurysm and superior vena cava fistula in an adult with a Blalock-Taussig shunt. Intensive Care Med 41: 1123-1124.
- Choi SY, Jin U, Suh JH, Kim YH (2008) Chronic post-traumatic pseudo aneurysm of the in nominate artery with an associated bovine aortic arch resulting in airway obstruction. Eur J Cardiothoracic Surg 34:669.
- Petrocheilou G, Kokkinis C, Stathopoulou S, Fragopoulou L, Mihos P, et al. (2008) Iatrogenic pseudo aneurysm of the brachiocephalic artery: a rare complication of Hickman line insertion. Int Urol Nephrol 40:1107-1110.
- Azarcon F, GhalebM (2014) Early diagnosis and treatment of a posttraumatic pseudo aneurysm/dissection of the innominate artery. Prehosp Disaster Med 29:209-211.
- Safran B, Garg K, ScherL, Shariff S, Lipsitz E (2019) Repair of isolated innominate artery pathology with a modified endovascular graft. Ann Vasc Surg 60: 475.
- Ito H, Yamamoto K, Hiraiwa T (2007) Spontaneous innominate artery perforation presenting as hemoptysis. Gen Thorac Cardiovasc Surg 55:73-75.
- Walter J, Hofmann WJ, Ugurluoglu A, Magometschnigg H (2003) False aneurysm after balloon dilation of a calcified innominate artery stenosis. J Endovasc Ther 10:825-828.
- Sakamoto H, Hattori T, Watanabe Y, Sakakibara Y (2006)Chronic post-traumatic pseudo aneurysm of the brachiocephalic artery with tracheal obstruction resulting in repeated pneumonia. Ann Thorac Surg 82:1101-1103.
- Reddi AA, Munasur MM, Naidoo RR, Steer DD (2005) Traumatic innominate artery aneurysm 26 years after stab injury. Ann Thorac Surg 79:1034-1036.
- Sladojevic M, Markovic M, Ilic N, Pejkic S, Banzic I, et al. (2016) Open treatment of blunt injuries of supra-aortic branches: case series. Ann Vasc Surg 31:205.
- Pruitt A, Dodson TF, Najibi S, Thourani V, Sherman A, et al. (2002) Distal septic emboli and fatal brachiocephalic artery mycoticpseudoaneurysm as a complication of stenting. J Vasc Surg 36:625-628.
- Kieffer E, Chiche L, Koskas F, Bahnini A (2001) Aneurysms of the innominate artery: Surgical treatment of 27 patients. J Vasc Surg 34:222-228.
- Cothren CC, Moore EE (2005) Postraumatic in nominate artery pseudo aneurysm. J Am Coll Surg 201:806-807.
- Greene JM, Cannon JW, Clouse WD, Pratt JW (2014) Fragmentation injury to the innominate artery in a three-year-old child. Ann Thorac Surg 97:1788-1790.
- Mousa AY, Batsides GP, Vogel TR (2010) Delayed presentation of traumatic innominate artery injury. J Vasc Surg 51:1014.
- Roan J, Luo C, Tsai H, Hu Y, Yang Y, et al. (2013)Surgical treatment of pseudo aneurysm of in nominate artery infected with burkholderia pseudomallei. Acta Cardiol Sin 29:98-101.
- Vanhuyse F, Maureira P, Amrein D, Villemot JP (2008) Brachiocephalic artery pseudo aneurysm caused by clavicle luxation. Eur J Cardiothoracic Surg 33: 111.
- Liu X, Miao Q, Liu J, Zhang C, Wang C, et al. (2014) In nominate pseudo aneurysm sub totally compressing the trachea as a result of blunt trauma. Ann Thorac Surg 97:1066-1068.
- Okubo K, Isobe J, Kitamura J, Ueno Y (2005) Mediastinitis and pseudo aneurysm of brachiocephalic artery long after the resection of invasive thymoma and postoperative irradiation. J Thorac Cardiovasc Surg 130:918-919.
- Moise MA, Hsu V, Braslow B, Woo YJ (2004) Innominate artery transection in the setting of a bovine arch. J Thorac Cardiovasc Surg 128:632-634.
- Pawar P, Rajendra N, Jagan J, Sukumar S, Raju R (2020) Tracheostomy creation leading to in nominate artery pseudo aneurysm: A case report. Indian J Anaesth 64:159-161.
- Augoustides JGT (2007) Anaesthetic management for endovascular repair of a giant in nominate artery pseudo aneurysm eroding into a mediastinal tracheostomy. Ann Cardiac Anaesth 10:155.
- Rispoli P, Varetto G, Savia F, Rinaldi M (2008) Large post-stenting in nominate artery pseudo aneurysm. Interact Cardiovasc Thorac Surg 7:444-446.
- Wells P, Estrera A (2005) Blunt traumatic in nominate pseudo aneurysm and left common carotid occlusion with an associated bovine aortic arch. J Thorac Cardiovasc Surg 130:928-929.
- Cordova AC, Bowen FW, Price LA, Dudrick SJ, Sumpio BE (2011) Traumatic in nominate artery pseudo aneurysm in the setting of a bovine arch. Ann Vasc Dis 4:252-255.
- Boutayeb A, Porcu P, Pirvu A, Chavanon O (2014) Post-traumatic injury of the brachiocephalic artery on pump beating heart repair. Heart Lung Circ 23:e226-e228.
- Lovelock T, Cheng A, Negria J, Fitzgerald M (2020) Transection of the origin of the innominate artery: A rare sequela of blunt traumatic chest trauma. Trauma Case Rep 27:100307.
- Mosquera VX, Velasco C, Gulias D, Farina MM (2013) Traumatic brachiocephalic trunk pseudo aneurysm. J Card Surg 28:430-432.
- Rabus MB, Kiran B, Sunar H (2008) Pseudo aneurysm of brachiocephalic artery caused by blunt chest trauma. Thorac Cardiov Surg 56:301-303.
- Philip RR, Boston US, Ballweg JA, Goldberg SP, Knott-Craig CJ (2012) Iatrogenic pseudo aneurysm of the innominate artery in a neonate. J Card Surg 27:242-244.
- Durieux R, Lavigne JP, Sprynger M, Defraigne JO (2012) Mycotic in nominate artery pseudo aneurysm complicating mitral endocarditis. Eur J Cardiothoracic Surg 42:e89.
- Brahmbhatt PA, Modi FD, Roy TM, Byrd Jr RP (2014) Common carotid artery laceration and innominate artery pseudo-aneurysm following a percutaneous dilatational tracheostomy attempt. Respir Care 59: e153-e155.
- De Troia A, Tecchio T, Azzarone M, Biasi L, Piazza P, Salcuni FP (2011) Endovascular treatment of an in nominate artery iatrogenic pseudo aneurysm following subclavian vein catheterization. Vasc Endovasc Surg 45: 78-82.
- Szeto WY, Fairman RM, Acker MA, Skelly CL, Augoustides JG, et al. (2006) Emergency endovascular deployment of stent graft in the ascending aorta for contained rupture of in nominate artery pseudo aneurysm in a pediatric patient. Ann Thorac Surg 81:1872-1875.
- Kanwar M, Desai D, Joumaa M, Guduguntla V (2009) Traumatic brachiocephalic pseudo aneurysm presenting as stroke in a seventeen-year-old. Clin Cardiol 32: e43-e45.
- Rahimi M, Bath J (2015) Arm ischemia and posterior stroke in a patient with a pulsatile mass in the right upper side of chest. In nominate artery pseudo aneurysm. JAMA Surg 150:811-812.
- Ahmed I, Katsanos K, Ahmad F, Dourado R, Lyons O, et al. (2009) Endovascular treatment of a brachiocephalic artery pseudo aneurysm secondary to biopsy at mediastinoscopy. Cardiovasc Intervent Radiol 32:792-795.
- Axisa BM, Loftus IM, Fishwick G, Spyt T, Bell PR (2000) Endovascular repair of an innominate artery false aneurysm following blunt trauma. J Endovasc Ther 7: 245-250.
- Kooraki S, Grohmann J, Elshikh S, Urbach H, Meckel S (2016) Covered stents for exclusion of iatrogenic common carotid artery–internal jugular vein fistula and brachiocephalic artery pseudo aneurysm. J Neuro Intervent Surg 8:e31.
- Li X, Shu C, Li QM, Fang K, Li M, et al. (2018) In nominate artery bifurcation pseudo aneurysm repair by kissing stent-grafts technique: a case report. J Med Case Rep 12:352.
- Sibille JA, Harding JP, Ballast JK, Hooshmand M, Madjarov JM, et al. (2016) Endovascular repair of an in nominate artery pseudo aneurysm using the Valiant Mona LSA branched graft device. J Vasc Surg Cases Innov Tech 3:1-3.
- Fukuda K, Yokoi Y (2014) Covered stent implantation for anastomotic pseudo aneurysm of in nominate artery post total aortic arch replacement. Cardiovasc Interv Ther 29:372-375.
- Chandler TA, Fishwick G, Bell PR (1999) Endovascular repair of a traumatic innominate artery aneurysm. Eur J Vasc Endovasc Surg 18:80-82.
- ScantleburyDC, Alli OO, Joyce DL, Rihal CS (2012) Percutaneous device closure of a pseudo aneurysm arising from the junction of the innominate artery and the aorta. J Thorac Cardiovasc Surg 144:732-734.
- Huang C, Kao H (2008) Endovascular management of post-traumatic innominate arterytransection with pseudo-aneurysm formation. Catheter Cardiovasc Interv 72:569-572.
- Choufani C, Aoun O, Mlynski A, Boddaert G, De Kerangal X, et al. (2017) Endovascular treatment of brachiocephalic artery war-related injury. Acta Chir Belg 117: 256-259.
- Volpe P, De Caridi G, Serra R, Alberti A, Massara M (2019) Successfully kissing stent of innominate artery and left common carotid artery subsequent to blunt injury, in the setting of a bovine aortic arch. Ann Vasc Surg 64:410e7.
- Zoffoli G, Saccani S, Larini P, Colli A, Gherli T (2006) Endovascular treatment of traumatic aortic dissection and in nominate artery pseudo aneurysm. J Trauma 61:447-450.
- Maddali MM, Badur RS, Rajakumar MC, Valliattu J (2006) Pseudo aneurysm of the in nominate artery: a delayed iatrogenic complication after internal jugular vein catheterization. J Cardiothoracic Vasc Anesth 20:853-855.
- Wang P, Yen P, Shyr M, Chen T, Chen A, et al. (2009) Endovascular repair of tracheo-innominate artery fistula. Acta Anaesthesiol Taiwan 47:36-39.
- Huttl K, Nemes B, Simonffy A, Entz L, Bérczi V (2002) Angioplasty of the innominate artery in 89 patients: experience over 19 years. Cardiovasc Intervent Radiol 25:109-114.
- Paukovits TM, Lukács L, Bérczi V, Hirschberg K, Nemes B, et al. (2010)Percutaneous endovascular treatment of innominate artery lesions: a single-centre experience on 77 lesions. Eur J Vasc Endovasc Surg 40:35-43.
- Aziz F, Gravett MH, Comerota AJ (2011) Endovascular and open surgical treatment of brachiocephalic arteries. Ann Vasc Surg 25: 569-581.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences