Endovascular Repair of Visceral Artery Aneurysms Decreases Length of Hospitalization
Miller SM, Sumpio BJ, Miller MS, Erben Y, Cordova AC and Sumpio BE
DOI10.21767/2573-4482.18.03.21
Miller SM1, Sumpio BJ1, Miller MS2, Erben Y1, Cordova AC3 and Sumpio BE1*
1Department of Surgery, Yale School of Medicine, New Haven, Connecticut, United States
2Icahn School of Medicine at Mt. Sinai, New York, United States
3Warren Alpert Medical School at Brown University, Providence, Rhode Island, United States
- *Corresponding Author:
- Bauer E Sumpio
Section of Vascular and Endovascular Surgery
Yale University School of Medicine
330 Cedar Street, BB204 New Haven
Connecticut 06510, USA
Tel: 203-785-2561
Fax: 203-785-7556
Email : bauer.sumpio@yale.edu
Received Date: September 24, 2018; Accepted Date: October 14, 2018; Published Date:October 21, 2018
Citation: Miller SM, Sumpio BJ, Miller MS, Erben Y, Cordova AC, et al. (2018) Endovascular Repair of Visceral Artery Aneurysms Decreases Length of Hospitalization. J Vasc Endovasc Therapy. 3:21.
Abstract
Objectives: Endovascular repair (EV) of visceral artery aneurysms (VAA) has risen. This study evaluates the outcomes of EV and open repair (OR) of VAA and compares splenic (SAA) to non-splenic (nsVAA) aneurysms.
Methods: Patients with VAA were identified from the Nationwide Inpatient Sample between 2003 and 2012. Demographics, comorbidities, complications and surgical outcomes of patients treated with EV and OR were compared. Patients were stratified into SAA and nsVAA and the same variables were compared between groups. Our primary end point was morbidity and mortality for treatment of VAA and the secondary end point was length of hospital stay after treatment.
Results: We identified 2561 VAA patients (1239 with SAA). The diagnosis of VAA rose throughout the study period. Patients treated for VAA rose from 62.8% in 2003 to 73.0% in 2012 (p<0.05). Of patients treated, those with EV rose from 26.8% in 2003 to 71.4% in 2012 (p<0.001) while those treated with OR fell from 73.2% to 28.6% (p<0.001). Mortality was not different between EV (3.8%) and OR (4.7%). Patients with OR experienced more postoperative complications (13.0% vs 9.7%, p<0.001). LOS was shorter for patients with EV (6.60 days vs 8.68, p<0.001).
Conclusion: The rate of EV repair for VAA has increased while OR is being used less. Mortality rates were similar for EV and OR. Patients with EV repairs experienced fewer complications and stayed two days shorter in the hospital than those with OR. SAA patients were younger, more likely to be female and had shorter LOS than nsVAA patients. With the increasing prevalence of VAA, EV procedures may be increasing the number of patients with VAA that can be treated.
Keywords
Visceral artery aneurysm; Splenic artery aneurysm; Mortality; Length of stay; Postoperative complications
Introduction
Visceral artery aneurysms (VAA) can be life-threatening conditions with high incidence of rupture and hemorrhage [1,2]. VAA includes aneurysms of the hepatic, celiac, superior mesenteric, gastric, gastroepiploic, pancreaticoduodenal, gastroduodenal, inferior mesenteric and in some circles, splenic arteries [3]. Greater availability and increased use of advanced imaging technology including computed tomography, magnetic resonance, ultrasonography, and arteriography have led to the increased incidental detection and classification of asymptomatic VAA [1,2,4]. As a result, VAAs have become increasingly frequent diagnoses confronting the general surgeon.
Visceral artery aneurysms carry an incidence of 0.1%-2% in the general population. Up to one-third of patients with VAAs will have multiple aneurysms [5].
There has been one natural history study to date that has calculated a growth rate of VAA to be 0.064 ± 0.18 cm/year [6]. VAAs have been reported to present as clinical emergencies in 19% of cases, but these data have not been able to be reproduced [7]. Depending on the size and the location, rupture of these lesions may be associated with a 25%-70% mortality rate [2].
The pathogenesis of VAA is poorly characterized. A variety of causative factors have been identified, including atherosclerosis (32% of cases), medial degeneration/segmental mediolysis (24%), abdominal trauma (22%), infection and inflammatory disease (10%), connective tissue disorders (Marfan syndrome, Ehlers- Danlos syndrome, Osler-Weber-Rendu disease), fibromuscular dysplasia, Kawasaki’s disease, hereditary hemorrhagic telangiectasia), and hyperflow conditions (portal hypertension, pregnancy) [8]. Atherosclerosis has been suggested to be the etiology for as many as 61% of VAA [9]. Incidence of VAAs differs between men and women, with SAAs more common in multiparous women and hepatic and gastroduodenal artery aneurysms more common in men [10].
There is currently no standardized consensus regarding the indications for treatment of VAA, making the course of action for such an incidental finding difficult to determine. Generally speaking, VAAs are treated if symptomatic, are larger than 2 cm in a good-risk surgical candidate, have a rapid growth of more than 0.5 cm/year, when present in a pregnant women or those of childbearing age, or in patients undergoing an orthotopic liver transplantation [7,11]. However, the size of the VAA has not been shown to be correlated to its risk of rupture [12].
Over the past decade, there has been steady increase in the utilization of minimally invasive interventions for vascular occlusive and aneurysmal disease. These less invasive methods allow more patients to be candidates for surgery, and we have seen an increase in the percentage of patients with VAA who are treated surgically. The purpose of this study was to compare EV to open therapy in regards to patient characteristics and postoperative outcomes. Since splenic artery aneurysms (SAA) have different demographic and clinical characteristics, VAA patients were stratified into those with SAA and those with nonsplenic artery aneurysms (nsVAA) and compared based on the same parameters.
Methods
Patients admitted with a primary diagnosis of VAA from 2003- 2012 were identified from the Healthcare Utilization Project (HCUP) Nationwide Inpatient Sample (NIS). Developed by the Agency for Health Research and Quality (AHRQ), the NIS represents the largest all-payer publicly available dataset in the United States, containing approximately 10 million discharges annually across the United States [13]. All investigators with access to the data have completed online training and certified Data User Agreements with HCUP. This study includes completely de-identified data and it was approved as exempt from review by the Yale Human Investigations Committee. Therefore, informed consent was not obtained from participants.
Patient selection
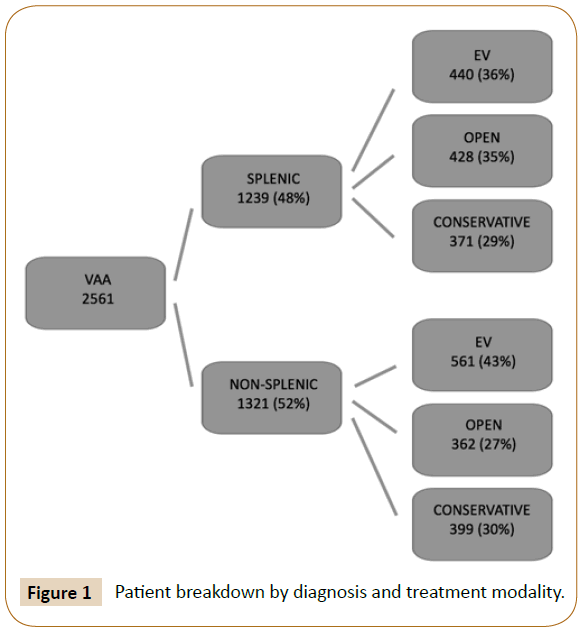
The NIS includes up to twenty-five International Classification of Disease, Ninth revision, Clinical Modification (ICD-9) diagnosis and fifteen ICD-9-CM procedural codes. Patients were included if they had a primary diagnosis code of ‘other visceral artery aneurysm’ (ICD-9-CM 442.84) or ‘splenic artery aneurysm’ (ICD- 9-CM 442.83). Patients were excluded if they had concordant aortic pathology (ICD-9-CM 441, 441.0, 441.1, 441.2, 441.3, 441.4, 441.5, 441.6, 441.7, 441.9, 441.00, 441.01, 441.02, 441.03). Procedure codes were queried to determine the type of treatment that each patient received. Patients were classified as having open repair (ICD-9-CM 38.06, 38.16, 38.36, 38.46, 38.66, 38.86, 39.26, 39.50, 39.52, 39.59), endovascular repair (ICD-9-CM 39.71, 39.79, 39.90), or no intervention (conservative management). Admissions involving both endovascular and open procedures represent either hybrid procedures or open surgery after failed endovascular intervention, and they were included in the open group for outcomes analysis (Figure 1).
Diagnosis codes were queried for the presence of the following comorbidities: coronary artery disease (ICD-9-CM 414.00, 414.01), hypertension (ICD-9-CM 401.0, 401.9), dysrhythmia (ICD-9-CM 427.0, 427.1, 427.2, 427.3, 427.31, 427.32, 427.4, 427.41, 427.42, 427.5, 427.6, 427.60, 427.61, 427.69, 427.8, 427.81, 427.89, 427.9), atrial fibrillation (ICD-9-CM 427.31), prior myocardial infarction (ICD-9-CM 412), congestive heart failure (ICD-9-CM 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, 428.9), chronic obstructive pulmonary disease (ICD-9-CM 490, 491, 492, 492.0, 492.8, 493, 493.0, 493.1, 493.2, 494, 495, 496, 496.0), chronic renal failure (ICD-9-CM 585.1, 585.2, 585.3, 585.4), peripheral vascular disease (ICD-9-CM 443.9).
Primary and secondary endpoints
Our primary end-points included mortality (ICD-9-CM 798.1), cardiac complications (ICD-9-CM 410.0-410.9, 997.1, 998.0), respiratory complications (ICD-9-CM 415.1, 997.3), peripheral vascular complications (ICD-9-CM 997.2), wound complications (ICD-9-CM 998.3, 998.31, 998.32, 998.83), infectious complications (ICD-9-CM 998.5, 998.51, 998.59, 999.3), acute renal failure (ICD-9-CM 584.5-584.9, 997.5) and hematologic complications (ICD-9-CM 453.40, 453.41, 453.42, 453.81, 453.82, 453.83). The secondary endpoint was length of hospital stay (LOS) from index admission until discharge alive.
Statistical analysis
Baseline characteristics were described as counts and percentages (dichotomous variables) or means and standard deviations (continuous variables). Differences at baseline were assessed using Pearson χ2 or Fisher exact testing and Student’s t-test, where appropriate. Logistic regression analysis was performed to identify predictors of in-hospital mortality. Statistical significance was set at a p-value of 0.05. All statistical analysis was performed using SPSS v. 24.0 (IBM Corp., Armonk, NY, USA).
Results
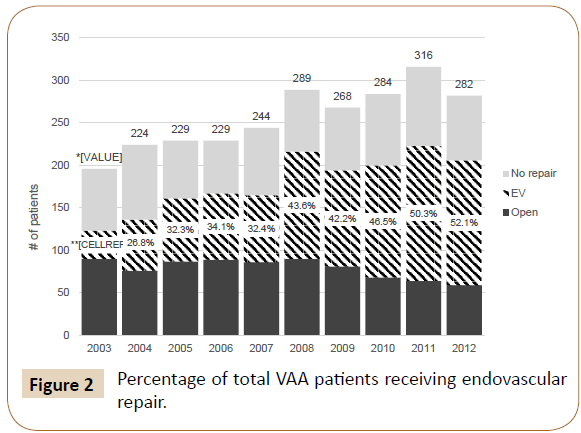
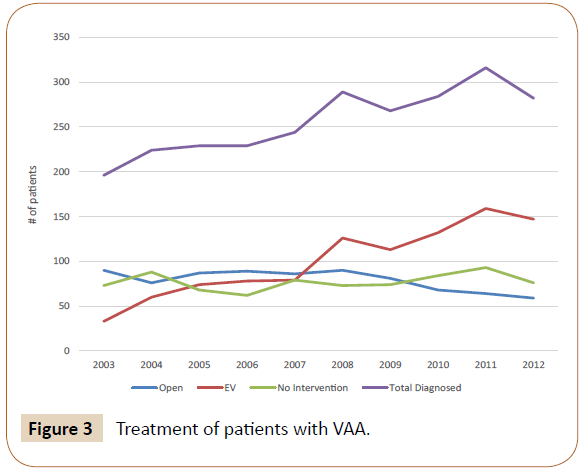
We identified 2561 patients with a primary diagnosis of VAA between 2003 and 2012. 1239 patients were diagnosed with SAA and the other 1322 with aneurysm of another visceral artery. In total, there were 1001 patients treated endovascularly, 790 patients treated with open repair, and 770 who did not receive surgical intervention. The number of VAA diagnoses increased from 196 in 2003 to 282 in 2012 (Figure 2). The percentage of patients receiving surgical intervention increased from 62.8% in 2003 to 73.0% in 2012 (p<0.05). Those who were managed medically without surgery decreased from 37.2% to 27.0% (p<0.05). Of the patients receiving treatment, those with EV repair rose from 26.8% to 71.4% (p<0.001) in the time period examined, while those treated with OR decreased from 73.2% to 28.6% (p<0.001) (Figure 3).
EV vs. OR
Patients receiving EV repair and those having OR were similar in age (58.7 years vs. 59.1 years, ns) and gender (51.1% female vs. 50.0% female, ns) (Table 1). Cardiac dysrhythmia was more prevalent in those having open repair (15.8% vs. 12.1%, p<0.05) and chronic renal failure was more common in those undergoing endovascular intervention (1.4% vs. 0.01%, p<0.05). There were no differences in coronary artery disease, hypertension, atrial fibrillation, prior myocardial infarction, heart failure, chronic obstructive pulmonary disease, or peripheral vascular disease.
| Demographics | VAA (N=2561) | Treated | Untreated | p-value | |
|---|---|---|---|---|---|
| EV (N=1001) | open (N=790) | (N=770) | open vs. EV | ||
| Age (years) | 58.7 | 58.7 | 59.1 | 58.1 | NS |
| Female | 51.10% | 51.10% | 50.00% | 53.50% | NS |
| Coronary artery disease | 9.60% | 8.80% | 9.90% | 10.40% | NS |
| Hypertension | 42.40% | 40.90% | 44.70% | 42.20% | NS |
| Dysrhythmia | 13.30% | 12.10% | 15.80% | 12.30% | <0.05 |
| Atrial fibrillation | 8.30% | 8.40% | 8.50% | 7.90% | NS |
| Prior myocardial infarction | 3.00% | 2.50% | 3.20% | 3.60% | NS |
| Heart failure | 5.00% | 5.60% | 4.30% | 5.10% | NS |
| Chronic obstructive pulmonary disease | 7.30% | 7.10% | 1.10% | 7.40% | NS |
| Chronic renal failure | 0.90% | 1.40% | 0.10% | 1.00% | <0.05 |
| Peripheral vascular disease | 1.80% | 1.50% | 1.90% | 2.10% | NS |
| Complications | |||||
| Mortality | 4.30% | 3.80% | 4.70% | 4.40% | NS |
| Cardiac complications | 1.50% | 0.90% | 2.90% | 0.80% | <0.05 |
| Respiratory complications | 1.30% | 0.30% | 2.80% | 1.20% | <0.001 |
| Peripheral vascular complications | 0.40% | 0.90% | 0.00% | 0.10% | <0.05 |
| Wound complications | 0.30% | 0.00% | 0.60% | 0.30% | <0.05 |
| Infectious complications | 1.30% | 0.80% | 2.40% | 0.90% | <0.05 |
| Acute renal failure | 5.30% | 6.60% | 5.40% | 3.60% | NS |
| Hematologic complications | 1.00% | 1.40% | 0.80% | 0.80% | NS |
| Any complication | 9.90% | 9.70% | 13.00% | 7.00% | <0.05 |
| LOS (days) | 7.1 | 6.6 | 8.7 | 6.1 | <0.001 |
Table 1 VAA patient demographics and surgical outcomes.
There was no difference in mortality between patients who had received EV repair and those with OR (3.8% vs. 4.7%, ns). Patients with OR were more likely to experience complications (13.0% vs. 9.7%, p<0.05). More specifically, they showed higher rates of cardiac complications (2.9% vs. 0.90%, p<0.05), respiratory complications (2.8% vs. 0.30%, p<0.001), wound complications (0.60% vs. 0%, p<0.05), and infectious complications (2.4% vs. 0.80%, p<0.05). In contrast, EV patients were more likely to experience peripheral vascular complications (0.90% vs. 0%, p<0.05). Length of stay (LOS) was shorter for patients with EV repair (6.6 days vs. 8.7 days, p<0.001).
Regression analysis revealed that the type of repair was not an independent predictor of inpatient mortality. Significant predictors of inpatient mortality included dysrhythmia (OR, 2.62; 95% CI 1.48-4.64, p<.001) and infectious complications (OR, 3.76; 95% CI 1.17-12.01, p<.05).
SAA vs. nsVAA
SAA patients were younger (56.7 years vs. 60.5, p<0.001) and more likely to be female (62% vs. 45%, p<0.001) than those with nsVAA. These patients were also less likely to have coronary artery disease (8.4% vs. 11.5%, p<0.05), hypertension (39.8% vs. 47.9%, p<0.05), and COPD (5.8% vs. 9.3%, p<0.05) (Table 2). A higher percentage of nsVAA patients were treated with endovascular repair (42.4% vs. 35.5%, p<0.001), while more SAA patients received open repair (34.5% vs. 27.4%, p<0.001). There was no difference in the number of patients managed nonoperatively. There were no significant differences in mortality or rates of surgical complications aside from SAA patients having more respiratory complications (2.1% vs. 0.6%, p<0.05). SAA patients had shorter LOS (6.3 days vs. 7.9, p<0.001).
| Demographics | VAA (N=2561) | SAA (N=1239) | nsVAA (N=1322) | p-value |
|---|---|---|---|---|
| Age (years) | 58.7 | 56.7 | 60.5 | <0.001 |
| Female | 52.00% | 61.50% | 44.80% | <0.001 |
| Coronary artery disease | 9.60% | 8.40% | 11.50% | <0.05 |
| Hypertension | 42.40% | 39.80% | 47.90% | <0.05 |
| Dysrhythmia | 13.30% | 12.00% | 15.60% | NS |
| Atrial fibrillation | 8.30% | 7.30% | 9.90% | NS |
| Prior myocardial infarction | 3.10% | 2.70% | 3.60% | NS |
| Heart failure | 5.00% | 4.50% | 5.90% | NS |
| Chronic obstructive pulmonary disease | 7.30% | 5.80% | 9.30% | <0.05 |
| Chronic renal failure | 0.90% | 0.70% | 1.10% | NS |
| Peripheral vascular disease | 1.80% | 1.50% | 2.30% | NS |
| Complications | ||||
| EV repair | 39.10% | 35.50% | 42.40% | <0.001 |
| Open repair | 30.90% | 34.50% | 27.40% | <0.001 |
| Conservative management | 30.10% | 29.90% | 30.20% | NS |
| Mortality | 4.00% | 3.90% | 4.60% | NS |
| Cardiac complications | 1.50% | 1.40% | 1.60% | NS |
| Respiratory complications | 1.30% | 2.10% | 0.60% | <0.05 |
| Peripheral vascular complications | 0.40% | 0.50% | 0.30% | NS |
| Wound complications | 0.30% | 0.10% | 0.50% | NS |
| Infectious complications | 1.30% | 1.50% | 1.10% | NS |
| Acute renal failure | 5.40% | 4.80% | 5.90% | NS |
| Hematologic complications | 1.00% | 0.70% | 1.30% | NS |
| Any complication | 9.90% | 9.70% | 10.00% | NS |
| LOS (days) | 7.1 | 6.3 | 7.9 | <0.001 |
Table 2 SAA vs. nsVAA.
Regression analysis revealed that the type of aneurysm (SAA vs. nsVAA) was not an independent predictor of inpatient mortality. Significant predictors of inpatient mortality included hypertension (OR, 0.38; 95% CI 0.22-0.66, p<.05) and COPD (OR, 2.11; 95% CI 1.03-4.32, p<.05).
Discussion
Our analysis indicates that there has been an increase in patients diagnosed with VAA between 2003 and 2012. Along with this increase, the rates of surgical intervention and specifically, EV intervention have risen as well. The increasing popularity of EV therapy has been documented in the treatment of aortic aneurysms [14,15], hepatic aneurysms [15], and renal aneurysms [16,17]. This trend is likely due, in part, to the larger cohort of patients that are candidates for EV therapy as opposed to OR and to the excellent outcomes seen with EV therapy [11,18- 20]. Previous studies have shown that patients with significant comorbidities who were previously denied open aortic aneurysm repair based on perioperative risk are now eligible for EV repair and are being referred to surgeons [18,21]. It is likely that this same trend exists for the repair of VAA, as this analysis shows an increase in patients admitted with a primary diagnosis of VAA as well as an increase in the number of patients treated with EV intervention, while the rate of conservative management and OR have decreased.
Medical comorbidities were similar between the EV and OR cohorts. There were no differences in the rates of coronary artery disease, hypertension, atrial fibrillation, prior myocardial infarction, heart failure, COPD and peripheral vascular disease. Similar patient demographics have been seen in EV to OR comparisons of VAA patients in previous studies [22]. In addition, research focused on abdominal aortic aneurysms (AAA) have shown that comorbidities are similar amongst patients receiving EV and open aneurysm repair [23,24]. Analysis revealed that preoperative dysrhythmias were more common in patients who would have OR, while chronic renal failure was more common in the cohort that opted for EV treatment.
Mortality was similar between the two patient groups examined. This finding contradicts those found in studies of patients having AAA repair where mortality was found to be higher in those receiving open repair [25-27]. However, Hislop et al. report similar mortality with EV and open patients receiving renal artery aneurysm repair [16].
Patients with OR were more likely to experience a complication after surgery. Specifically, they had higher rates of cardiac, respiratory, wound, and infectious complications. Similar trends with higher complication rates after open repair were seen after repair of AAA [25-27], while postoperative complication rates were similar between EV and OR of renal artery aneurysms [16]. Length of postoperative hospital stay was found to be shorted for EV patients by about two days. This trend has been demonstrated in similar comparisons of AAA [25-27] and renal artery aneurysm repairs [16].
Since SAA are considered by some to be different than the nsVAA, we divided our VAA patients into those two groups. We demonstrate that SAA patients were more likely to be female than their nsVAA counterparts. This sex discrepancy is similar those seen in previous literature [28,29], and may be explained by the hormonal effects on the arterial wall [29]. These SAA patients were also younger than those with aneurysms of other visceral arteries by almost four years. This may be explained by the common presentation of symptomatic SAA in pregnancy, resulting in young women comprising a significant portion of the SAA cohort [28,30]. nsVAA patients were more likely to have coronary artery disease, hypertension and COPD than those with SAA. The increased prevalence of these comorbidities is likely attributable to the nsVAA cohort being older than the SAA [31,32].
nsVAA patients were more likely to receive endovascular therapy, while those with SAA had higher rates of open repair. There were no differences in mortality between SAA and nsVAA patients and postoperative complication rates were similar except for a higher rate of respiratory complications in SAA patients. Length of stay was shorter for SAA patients by about 1.5 days.
This study has several limitations. The HCUP NIS database is based on billing codes and as such, we were not able to assess the complexity of the presenting visceral artery aneurysms. ICD- 9-CM codes do not describe the size or specific location of the aneurysms in question. As a result, we could not evaluate the potential relationship between aneurysm location and the choice between EV versus OR.
It should also be noted that the NIS database is a compilation of hospital admissions. Thus, our analysis does not include outpatient aneurysm repair. Outpatient elective aneurysm repair has become more common with the increasing popularity of endovascular therapy [33,34]. In addition, we are unable to comment on potential differences in long-term outcomes between EV and open interventions based on the lack of ability to access readmission data. Further research is needed to evaluate the utility of EV therapy in regards to re-intervention rates and follow up requirements.
Conclusions
EV technique is becoming more widely used in the repair of VAAs, while the use of open repair has decreased. There was no difference in mortality between the two cohorts. EV patients had shorter LOS and lower rates of complications. SAA patients were younger, more likely to be female, and more likely to receive EV intervention than those with nsVAA.
References
- Cordova AC, Sumpio BE (2013) Visceral artery aneurysms and pseudoaneurysms-should they all be managed by endovascular techniques?. Ann Vasc Dis 6:687-93.
- Ferrero E, Viazzo A, Ferri M, Robaldo A, Piazza S, et al. (2011) Management and urgent repair of ruptured visceral artery aneurysms. Ann Vasc Surg 25: 981.e7-981.e11.
- Ibrahim F, Dunn J, Rundback J, Pellerito J, Galmer A (2018) Visceral artery aneurysms: Diagnosis, surveillance, and treatment. Curr Treat Options Cardiovasc Med 20:97.
- Abdel Razek AAK, Albair GA, Samir S (2017) Clinical value of classification of venous malformations with contrast-enhanced MR Angiography. Phlebol J Venous Dis 32:628-33.
- Busuttil RW, Brin BJ (1980) The diagnosis and management of visceral artery aneurysms. Surgery 88:619-624.
- Erben Y, Brownstein AJ, Rajaee S, Li Y, Rizzo J, et al. (2017) PC084 Natural history and management of splanchnic artery aneurysms in a single tertiary referral center. J Vasc Surg 65:161S-162S.
- Ferrero E, Ferri M, Viazzo A, Robaldo A, Carbonatto P, et al. (2011) Visceral artery aneurysms, an experience on 32 cases in a single center: treatment from surgery to multilayer stent. Ann Vasc Surg 25:923-935.
- Gehlen JMLG, Heeren PAM, Verhagen PF, Peppelenbosch AG (2011) Visceral artery aneurysms. Vasc Endovascular Surg 45: 681-687.
- Regus S, Lang W (2016) Rupture risk and etiology of visceral artery aneurysms and pseudoaneurysms. Vasc Endovascular Surg 50:10-15.
- Abbas MA, Stone WM, Fowl RJ, Gloviczki P, Oldenburg WA, et al. (2002) Splenic artery aneurysms: Two decades experience at mayo clinic. Ann Vasc Surg 16:442-449.
- Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, et al. (2007) The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 45:276-283.
- Pitton MB, Dappa E, Jungmann F, Kloeckner R, Schotten S, et al. (2015) Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur Radiol 25: 2004-2014.
- www.hcup-us.ahrq.gov/nisoverview.jsp
- Kalra K, Arya S (2017) A comparative review of open and endovascular abdominal aortic aneurysm repairs in the national operative quality improvement database. Surgery 162: 979-988.
- Mohan IV, Stephen MS (2013) Peripheral arterial aneurysms: Open or endovascular surgery?. Prog Cardiovasc Dis 56:36-56.
- Hislop SJ, Patel SA, Abt PL, Singh MJ, Illig KA (2009) Therapy of renal artery aneurysms in new york state: Outcomes of patients undergoing open and endovascular repair. Ann Vasc Surg 23:194-200.
- Buck DB, Curran T, McCallum JC, Darling J, Mamtani R, et al. (2016) Management and outcomes of isolated renal artery aneurysms in the endovascular era. J Vasc Surg 63:77-81.
- Arko FR, Filis KA, Seidel SA, Gonzalez J, Lengle SJ, et al. (2004) How many patients with infrarenal aneurysms are candidates for endovascular repair? the northern california experience. J Endovasc Ther 11:33-40.
- Schumacher H, Allenberg JR, Eckstein HH (1996) Morphological classification of abdominal aortic aneurysm in selection of patients for endovascular grafting. Br J Surg 83:949-950.
- Prinssen M, Verhoeven ELG, Buth J, Cuypers PWM, van Sambeek MRHM, et al. (2004) A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 351:1607-1618.
- Lederle FA (1996) Risk of rupture of large abdominal aortic aneurysms. Disagreement among vascular surgeons. Arch Intern Med 156:1007-1009.
- Saltzberg SS, Maldonado TS, Lamparello PJ, Cayne NS, Nalbandian MM, et al. (2005) Is endovascular therapy the preferred treatment for all visceral artery aneurysms?. Ann Vasc Surg 19:507-515.
- Bae M, Chung SW, Lee CW, Song S, Kim E, et al. (2017) A comparative study of abdominal aortic aneurysm: Endovascular aneurysm repair versus open repair. Korean J Thorac Cardiovasc Surg 50:263-269.
- Greenhalgh R (2004) Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet 364: 843-848.
- Behrendt C-A, Sedrakyan A, Rieß HC, Heidemann F, Kölbel T, et al. (2017) Short-term and long-term results of endovascular and open repair of abdominal aortic aneurysms in Germany. J Vasc Surg 6: 1704-1711.
- Johnson ML, Bush RL, Collins TC, Lin PH, Liles DR, et al. (2006) Propensity score analysis in observational studies: Outcomes after abdominal aortic aneurysm repair. Am J Surg 192:336-343.
- Bush RL, Johnson ML, Collins TC, Henderson WG, Khuri SF, et al. (2006) Open versus endovascular abdominal aortic aneurysm repair in va hospitals. J Am Coll Surg 202:577-587.
- Mattar SG, Lumsden AB (1995) The management of splenic artery aneurysms: Experience with 23 cases. Am J Surg 169: 580-584.
- Stanley JC, Fry WJ (1974) Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 76:898-909.
- Selo-Ojeme DO, Welch CC (2003) Review: Spontaneous rupture of splenic artery aneurysm in pregnancy. Eur J Obstet Gynecol Reprod Biol 109:124-127.
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, et al. (1995) Prevalence of hypertension in the us adult population results from the third national health and nutrition examination survey, 1988-1991. Hypertens 25:305-313.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, et al. (2006) Global burden of COPD: Systematic review and meta-analysis. Eur Respir J 28:523-532.
- Fankhauser GT, Stone WM, Naidu SG, Oderich GS, Ricotta JJ, et al. (2011) The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 53:966-970.
- Lachat ML, Pecoraro F, Mayer D, Guillet C, Glenck M, et al. (2013) Outpatient endovascular aortic aneurysm repair. Ann Surg 258:754-759.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences