Endovascular Treatment of an Aneurysm Rupture into the Inferior Vena Cava
Miltiadis Matsagkas*, Olga Xiropotamou, George Kouvelos, Michalis Peroulis, Vasilios Bouris, Maria Zeygara, Thomas Vadivoulis and Maria Argyropoulou and
DOI10.21767/2573-4482.10004
1Department of Radiology, School of Medicine, University of Ioannina, Ioannina, Greece
2Department of Surgery- Vascular Surgery Unit, School of Medicine, University of Ioannina, Ioannina, Greece
- *Corresponding Author:
- Miltiadis I Matsagkas
Professor of Vascular Surgery, Department of Surgery Vascular Surgery Unit Medical School
University of Ioannina, Ioannina University Campus, S. Niarchos Avenue
45110, Ioannina, Greece
Tel: +30 26 5100 7422
Fax: +30 26 51033379
Email: mimats@cc.uoi.gr; milmats@gmail.com
Received date: February 19, 2016; Accepted date: March 22, 2016; Published date: March 31, 2016
Citation: Xiropotamou O, Kouvelos G, et al. Endovascular Treatment of an Aneurysm Rupture into the Inferior Vena Cava Journal of Vascular & Endo Surgery. 2016, 1:1. doi: 10.21767/2573-4482.100004
Introduction
Major abdominal arteriovenous fistulas are defined as anomalous communications between the aorta, iliac or renal arteries and the inferior vena cava (IVC), iliac or renal veins. Aortocaval fistula (ACF) is one of the less well-recognized complications occurring in < 1% of all abdominal aortic aneurysms (AAAs) and 3% - 7% of all ruptured AAAs [1].
The overall mortality in patients with AAA and associated ACF has been reported as high as 67% [2]. This high rate could be partially attributed to the excessive blood flow through the fistula, usually resulting to central venous hypertension, refractory congestive heart failure and multi organ failure [1-3]. ACF can also present in combination with retroperitoneal rupture, having a much worse prognosis [2, 3].
Aortocaval fistula, if not treated, is rapidly fatal and repair of the arteriovenous communication is mandatory for restitution of a normal hemodynamic status. Herein we present a case of aortocaval fistula in a ruptured AAA (rAAA), focusing on diagnosis and treatment options.
Case Report
A 65 years old male with a history of hypertension and tobacco use presented to the emergency department complaining for an acute abdominal pain radiating to the lower spine. His condition was stable (blood pressure 135 / 80 mmHg, heart rate 100 beats / min, respiratory rate 24 / min).
Abdominal examination revealed a pulsatile mass and a continuous audible bruit with systolic accentuation over the central abdomen. No shortness of breath was noticed. Laboratory tests were normal.
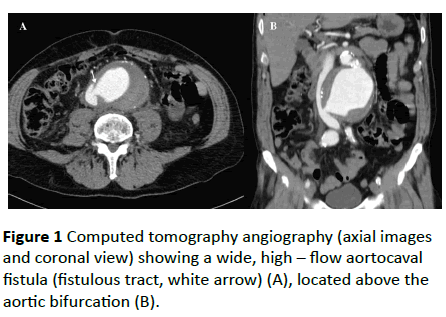
Urgent computed tomography (CT) angiography, revealed an infrarenal AAA 9, 3 cm in diameter. During the arterial phase, there was a rapid contrast filling of the inferior vena cava, indicating the presence of a dynamic fistula from the right lateral wall of the aneurysmal sac (Figure 1).
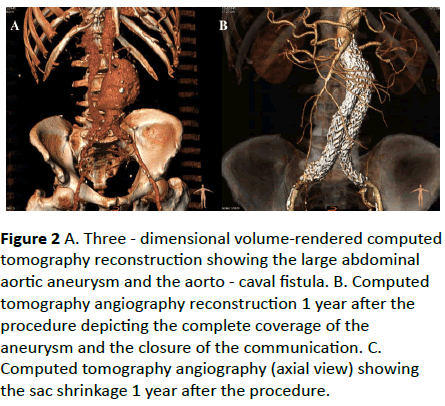
An endovascular repair of the rAAA was decided. Anatomical characteristics are presented in Table 1. An endograft with suprarenal fixation was chosen to accomplish better apposition to the highly angulated proximal aortic neck. After surgical cut down on both femoral arteries, the intraoperative aortogram confirmed the presence of the ACF without retroperitoneal leak. An Endurant II (Medronic Cardiovascular, Santa Rosa, CA, USA) aortic bifurcated stent graft was deployed. The total operating time was 90 minutes. The final aortogram revealed no endoleaks, exclusion of the aneurysm sac and complete sealing of the ACF.course was uneventful, and the patient discharged home on the fourth postoperative day. The CT scan at 12 months revealed the complete exclusion and shrinkage of the aneurysm sac, as well as the sealing of the fistula (Figure 2).
| Maximum AAA diameter | 90 mm |
| Aortic neck diameter | 26.8 mm-27.1 mm |
| Aortic neck length | 30.1 mm |
| Suprarenal angle | 49.4° |
| Infrarenal angle | 87.4° |
| Right common iliac artery | 30 mm |
| Left common iliac artery | 13.5 mm |
| AAA: Abdominal Aortic Aneurysm |
Table 1: Anatomical characteristic of the ruptured AAA.
Figure 2: A. Three - dimensional volume-rendered computed tomography reconstruction showing the large abdominal aortic aneurysm and the aorto - caval fistula. B. Computed tomography angiography reconstruction 1 year after the procedure depicting the complete coverage of the aneurysm and the closure of the communication. C. Computed tomography angiography (axial view) showing the sac shrinkage 1 year after the procedure.
Discussion
Prompt diagnosis of AAA with ACF is based mainly on symptoms and imaging studies and could occasionally be quite delayed as the signs of the fistula may be discrete. The probability of achieving a correct preoperative diagnosis remains quite low (0% - 50%) [4]. The “classical” clinical presentation includes 1) abdominal and / or back pain, 2) a pulsatile mass, and 3) an abdominal machinery bruit [4, 5]. Other symptoms comprise shock, lower limb edema, congestive heart failure, and scrotal edema or hematoma. Hematuria, oliguria, anuria and renal insufficiency are also frequently seen in cases of AAA with ACF. These symptoms are related to the large amount of blood shunted via the fistula. However, shock, compression of the IVC, or the presence of intra-aneurysmal thrombus at the site of the fistula can mask the classic symptoms, which may present in only 40% of cases [4]. It is interesting that although central venous pressure tends to be elevated in cases of AAA with ACF, it can sometimes be within the normal range or even lower [4].
The gold standard for AAA diagnosis is contrast - enhanced CT. CT images of AAA with ACF may exhibit several characteristics: a large aneurysm (over 8 cm in 75% of cases), compression of the IVC by the aneurysm, pelvic dilatation, and early opacification of the IVC during the arterial phase of contrast enhancement [4, 6]. The fistulous tract between AAA and IVC may be rarely detected, as it is shown in the present case [6]. Other imaging modalities such as ultrasonography, angiography, venography, magnetic resonance imaging, radioisotope studies, may be also used, but have not been widely established [4]. Ultrasonography has high sensitivity and specificity for diagnosing an AAA and in elective cases,color duplex ultrasonography can even detect the presence of an ACF without the use of a contrast agent. In cases of a ruptured AAA this diagnosis may be quite difficult and still remains unreliable; therefore it could be used only as an auxiliary approach at patient presentation [2].
Diagnosis and treatment before the development of shock can double the chances of survival from 25% to 50% [4]. Several management strategies have been reported including open surgery, hybrid approach, endovascular aortic repair, concomitant endovascular venous and aortic repair [3, 4]. Traditionally, open surgical repair was considered the first line treatment, though associated with high mortality (up to 21%) and morbidity [1, 5]. In the modern era where EVAR for a rAAA usually represents the first line choice for dealing with the aneurysmal disease, open repair is mainly restricted to cases when EVAR is not possible or even not available.
In order to reduce the morbidity and avoid the risks of open surgery caused by technically challenging dissection and bleeding, an endovascular approach has been introduced [3]. Endovascular stent grafting offers an attractive therapeutic alternative to the open repair of ACF as it does not involve a laparotomy, there is less blood loss and it might be done under local anesthesia. There are no specific protocols for the modern treatment of ACF with emergency EVAR, however the local algorithms for ruptured AAAs could be followed and the operation can be accomplished as a common rEVAR case.
In a systematic review Nakad et al. evaluated 48 articles on endovascular treatment of major abdominal arteriovenous fistulas including 54 patients, with the aortocaval segment being the most common fistula site [1]. Aortic stent grafts were used in 78% of patients, while technical success was 94%. Intraoperative mortality was 0% with a 90 days mortality of 10%, half of which were not related to the primary pathology. Of the successful procedures, 12% of patients had major complications that were successfully managed by endovascular therapy as well.
In the present case endovascular treatment has been achieved using aortic stent graft alone. Some authors consider this treatment as incomplete because it does not directly occlude ACF, maintaining the communication from cava to the excluded aneurysm sac and a high risk of type II endoleak [3]. However, successfully endovascular cava stent grafting has been described in the treatment of late endoleak type II after rEVAR in patients with ACF [7]. Hybrid strategy with initial covering of the caval entry using conventional stent grafts and subsequent open surgery for the aortic aneurysm has also been reported [8]. The lack of vena specific stent-grafts and the absence of long term follow up data cannot adequately support primary inferior vena stent-grafting. Nevertheless in the case of a persistent type II endoleak, stent grafting of the IVC can be safely performed as a secondary procedure, given the fact that the size of vena cava would have been reduced after the initial endovascular repair [9]. The closure of the aorto caval fistula with an Amplatzer as a first step before the deployment of the aortic endograft has been published before with satisfying results [10]. However the use of such a device raises the total cost of the procedure, is technically challenging and demands the availability of an Amplatzer occluder device in a 24 h urgent basis.s
Conclusion
Endovascular management of a ruptured abdominal aortic aneurysm with a subsequent aorto - caval fistula is an effective, minimally invasive therapeutic option that obviates the need for an open repair associated with high morbidity and mortality rates [9]. Prompt diagnosis is essential and could result in early treatment and a better clinical outcome.
References
- Nakad G, AbiChedid G, Osman R (2014) Endovascular treatment of major abdominal arteriovenous fistulas: a systematic review. Vasc Endovascular Surg48:388-395.
- Nakazawa S, Mohara J, Takahashi T, Koike N, Takeyoshi I (2014) Aortocaval fistula associated with ruptured abdominal aortic aneurysm. Ann VascSurg 28:e5-e9.
- SilveiraPG, GunhaJ RF, Lima GB, Franklin RN, Bortoluzzi CT, et al. (2014) Endovascular treatment of ruptured abdominal aortic aneurysm with aortocaval fistula based on aortic and inferior vena cava stent-graft placement. Ann VascSurg28:e1-e5.
- Brightwell RE, Pegna V, Boyne N (2013)Aortocaval fistula: current management strategies. ANZ J Surg83:31-35.
- Janczak D, Chabowski M, Szydelko T, Garcarek J (2014) Endovascular exclusion of a large spontaneous aortocaval fistula in a patient with a ruptured aortic aneurysm. Vascular22:202-205.
- Taheri MS, Haghighatkhah H, Pourghorban R, Hosseini A (2013)Multidetector Computed Tomography findings of abdominal aortic aneurysm and its complications: a pictorial review. EmergRadiol 20:443-451.
- Melas N, Saratzis A, Saratzis N, Lazaridis I, Kiskinis D (2011) Inferior vena cava stent-graft placement to treat endoleak associated with an aortocaval fistula. J EndovascTher18:250-254.
- Siepe M, Koeppe S, Euringer W, Schlensak C (2009)Aorto-caval fistula from acute rupture of an abdominal aortic aneurysm treated with a hybrid approach. J VascSurg49:1574-1576.
- Antoniou GA, Koutsias S, Karathanos C, Sfyroeras GS, Vretzakis G, et al. (2009) Endovascular stent-graft repair of major abdominal arteriovenous fistula: a systematic review. J EndovascTher16:514-523.
- Godart F, Haulon S, Houmany M, Francart C, Brevière GM, et al. (2005)Transcatheter closure of aortocaval fistula with the amplatzer duct occluder. J EndovascTher 12:134-137.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences