Feraheme-Enhanced Magnetic Resonance Angiography (FEMRA) vs. Digital Subtraction Angiography in the Diagnosis and Treatment of Peripheral Vascular Disease in Patients with Renal Insufficiency
John D Dortch, Mellena D Bridges, Gregory Frey, David Sella, W Andrew Oldenburg, Houssam Farres, Erin Smith, Haley Lanigan and Albert G Hakaim
DOI10.21767/2573-4482.100028
John D Dortch1, Mellena D Bridges2, Gregory Frey2, David Sella2, W Andrew Oldenburg1, Houssam Farres1, Erin Smith2, Haley Lanigan1 and Albert G Hakaim1*
1Division of Vascular and Endovascular Surgery, Department of Surgery, Mayo Clinic Florida, Florida, USA
2Department of Radiology, Mayo Clinic Florida, USA
- *Corresponding Author:
- Albert G Hakaim
Division of Vascular and Endovascular Surgery, Department of Surgery, Mayo Clinic Florida, 4500 San Pablo Rd, Jacksonville, Florida, USA.
Tel: 904-953-2077
E-mail: Hakaim.Albert@mayo.edu
Received Date: October 05, 2016; Accepted Date: November 07, 2016; Published Date: November 13, 2016
Citation: Dortch JD, Bridges MD, Frey G, et al. Feraheme-Enhanced Magnetic Resonance Angiography (FEMRA) vs. Digital Subtraction Angiography in the Diagnosis and Treatment of Peripheral Vascular Disease in Patients with Renal Insufficiency. J Vasc Endovasc Surg. 2016, 1:4. doi: 10.21767/2573-4482.100028
Abstract
Background: Ferumoxytol is an Ultrasmall Superparamagnetic Iron Oxide (USPIO) which has demonstrated promise as a novel contrast agent with an excellent safety profile in patients with CKD. Our aim was to evaluate the safety and utility of this agent for diagnosis and operative planning in the setting of PAD and CKD. A comparison with digital subtraction angiography is also provided.
Methods: Between April, 2013 and September, 2014 seven patients with renal insufficiency (CKD ≥ Stage 3) and symptomatic PAD underwent Fe-MRA. This was followed by DSA limited to the vascular bed with significant stenosis as detected by Fe-MRA. Renal function was assessed before and after each procedure. Images were qualitatively scored at the iliac, femoral, popliteal and tibial levels by two interventional radiologists. Degree of stenosis was also scored for comparison.
Results: Seven male patients were studied with a mean age of 75 years (64-92). 5 therapeutic procedures (4 endovascular and 1 open) were performed. There were no statistically significant differences in creatinine or GFR after Fe-MRA or DSA. Iodinated contrast load (mg I) for DSA was reduced in comparison with age and disease matched controls (8240 ± 5206 vs. 29320 ± 15605, p=0.001). No statistically significant differences were found for degree of stenosis or mean image quality score below the iliac level. There were no adverse events in response to Ferumoxytol administration.
Conclusion: Fe-MRA provides image quality and estimation of degree of stenosis comparable to DSA. Fe-MRA may serve as an alternative to CTA or gadoliniumbased MRA for patients with end-stage renal disease.
Keywords
Ferumoxytol; Feraheme; Angiography; Vascular imaging; Peripheral arterial disease; Chronic kidney disease
Introduction
The association between Peripheral Arterial Disease (PAD) and Chronic Kidney Disease (CKD) is well established with an estimated 7.4-24% of patients with stage ≥ 3 CKD carrying a clinical diagnosis of PAD [1]. Conversely, impaired renal function is prevalent among patients with PAD in the range of 27-36% [2,3]. This correlation poses a problem for vascular interventionists who rely heavily on contrast-based imaging for operative planning. Iodinated contrast agents are generally avoided in the setting of CKD due to the risk of contrast-induced nephropathy [4]. Magnetic resonance angiography using gadolinium- based agents provide high quality vascular imaging with less nephrotoxicity and a decreased adverse reaction profile. However, gadolinium-based agents are not used without risk in the setting of advanced CKD due to their association with Nephrogenic Systemic Fibrosis (NSF) [5].
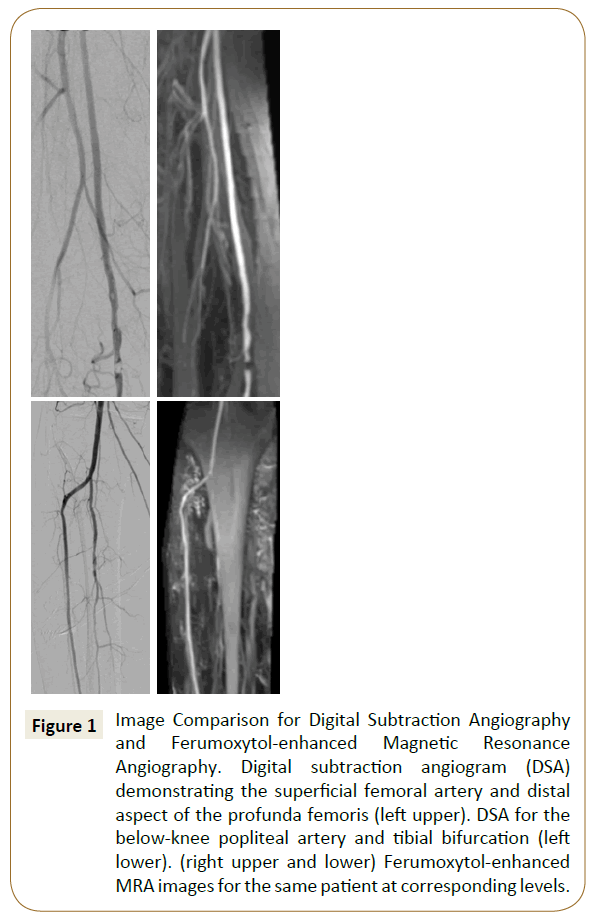
Over the past decade, Ultrasmall Superparamagnetic Iron Oxide (USPIO) agents have emerged as a novel alternative to gadoliniumbased contrast agents for MR angiography [6-8]. USPIO particles serve as blood pool contrast agents which offer the advantage of a longer image acquisition window and less surrounding soft tissue enhancement in comparison with extracellular gadolinium-based agents [9]. Ferumoxytol (Feraheme; AMAG Pharmaceuticals, Cambridge, MA) is a commercially available USPIO with FDA approval for the treatment of iron deficiency anemia in adult patients with CKD [10]. This carbohydrate-coated nanoparticle is cleared via the reticuloendothelial system with very little renal excretion, contributing to a low adverse event rate among patients with end-stage renal disease [11]. Its established safety profile makes ferumoxytol an attractive alternative contrast agent for peripheral vascular imaging in the setting of CKD. Though prior reports have analyzed the feasibility of ferumoxytol-enhanced MRA (Fe-MRA) for lower extremity vascular imaging [9,12], no data are currently available comparing image accuracy and quality with the gold-standard Digital Subtraction Angiography (DSA) using iodinated contrast. Our objective in this study was to compare image quality between Fe-MRA and DSA and determine the utility of Fe-MRA for reducing the contrast dose required during catheter-based peripheral interventions (Figure 1).
Figure 1: Image Comparison for Digital Subtraction Angiography and Ferumoxytol-enhanced Magnetic Resonance Angiography. Digital subtraction angiogram (DSA) demonstrating the superficial femoral artery and distal aspect of the profunda femoris (left upper). DSA for the below-knee popliteal artery and tibial bifurcation (left lower). (right upper and lower) Ferumoxytol-enhanced MRA images for the same patient at corresponding levels.
Methods
Study population
Between April, 2013 and September, 2014 seven patients from the Mayo Clinic Florida vascular surgery clinic with renal insufficiency (CKD Stage ≥ 3 as defined by GFR) and symptomatic lower extremity arterial disease were prospectively identified to undergo Fe-MRA of the abdomen, pelvis, and extremities. Electronic medical records were reviewed to obtain baseline demographic characteristics and indications for imaging. Fe-MRA was subsequently followed by selective DSA and percutaneous intervention when indicated. Pre-procedure serum Creatinine (Cr) and estimated Glomerular Filtration Rate (eGFR) were obtained at baseline prior to Fe-MRA. Repeat renal function laboratory studies were obtained following both Fe-M RA and DSA. This study was approved by Mayo Clinic Florida Institutional Review Board.
Magnetic resonance imaging protocol
Patient hydration consists of administration of intravenous 0.5 L normal saline, along with several cups of water as tolerated. After some experimentation with volunteers, and as suggested by early research [6,9], ferumoxytol dose for 1.5T imaging is 4 mg/kg up to a maximum of the 510 mg of elemental iron contained in a single use, 17 mL vial. At 3T, we have found that dose reduction to 2 mg/kg avoids the additional T2* effects at higher field strengths. In either case, the calculated dose is diluted in sufficient normal saline to produce a 60 mL dose total. Injection rate is 1-2 mL/sec, with the higher rate used for MRA, immediately followed by a 30 mL saline chase.
Patients are placed supine in the scanner bore, on a gantry capable of rapid longitudinal motion. Phased-array body and extremity coils are used to ensure uniform coverage from above the aortic bifurcation through the ankles. Imaging occurs in two distinct stages. In the first, inflow into the arteries of the calf and ankle, from above the level of the trifurcation, is assessed. Following a 1 mL timing bolus, 7 mL of the dilute ferumoxytol is administered and a rapid time-resolved MRA (TWIST) is triggered (TR/TE 2.5/0.89 ms, flip angle 25°, FOV 500, 6/8 partial Fourier, slice thickness 1.2 mm, slice resolution 61%, phase resolution 69%, temporal resolution 10 sec per each of 8 measures). Source and Maximum Intensity Projection (MIP) reconstruction images are saved to produce a time-resolved depiction of inflow and small artery filling. The second portion of the examination is triggered fluoroscopically at first blush in the infrarenal aorta and employs three-station MRA of pelvis, thighs, and calves following a 40 mL injection of dilute ferumoxytol at 2 mL/sec (TR/ TE 3.37/1.4 msec, flip angle 25°, quick fat suppression, FOV 500, 6/8 partial Fourier, slice thickness 1.5 mm, slice resolution 61%, phase resolution 69%). The remaining contrast is administered by trickle flow.
Patients are monitored for hypo/hypertension and other complications during and for 30 min after scan completion.
Digital subtraction angiography
Digital subtraction angiography was performed using Visipaque 320™ (GE Healthcare, Cork, Ireland) with contrast diluted to 50% or 25% concentration at the discretion of the operating surgeon. For each study patient selective angiography was performed based on areas of concern identified on Fe-MRA. All images obtained were saved for post-procedural comparison with Fe- MRA. Total contrast volume administered during the procedure was routinely recorded in the postoperative documentation. The contrast volume used was converted to total mg dose of iodine to control for varying dilution factors used during DSA. The decision to proceed with percutaneous intervention was made based on intraoperative findings. An overview of procedures performed and contrast administered is provided in Table 2. A retrospective review of patients randomly selected from the study period was performed to identify 30 control patients undergoing nonselective angiography with or without peripheral percutaneous intervention to compare contrast volume administration.
| Patient | Procedure Performed | Iodinated Contrast Dose (mg)a |
|---|---|---|
| 1 | Superficial femoral artery balloon angioplasty and stent placement | 5200 |
| 2 | Balloon angioplasty of tibioperoneal trunk | 12800 |
| 3 | Diagnostic angiogram | 1280 |
| 4 | Superficial femoral artery balloon angioplasty and stent placement | 16000 |
| 5 | Balloon angioplasty of superficial femoral artery | 11200 |
| 6 | Diagnostic angiogram | 4800 |
| 7 | Diagnostic angiogram | 6400 |
| aTotal volume of Iodine in milligrams was calculated from the total dose of contrast administered after dilution. Dilution of contrast was performed at the surgeon’s discretion. | ||
Table 2: Procedural intervention and volume of contrast administered for patients undergoing both feraheme-enhanced MRA and digital subtraction angiography (N=7).
Image analysis
Fe-MRA and selective-DSA images were independently analyzed using a standardized scoring system by two interventional radiologists at our institution. Images were rated at the femoral, popliteal and tibial level based on quality of study and degree of stenosis identified. Quality scores ranged from 1-4 with 1=nondiagnostic, 2=sufficient, 3=good, 4=very good. Degree of stenosis was scored as 1=no stenosis, 2=<50%, 3=51-70%, 4=71-99%, 5=occluded vessel.
Statistical comparison
Statistical comparison was performed with student’s t-test or ANOVA when appropriate. No adjustment for multiple testing was made in these exploratory analyses, and p values ≤ 0.05 were considered as statistically significant.
Results
Seven male patients with a mean age of 75 ± 4.1 were identified for the study. Six patients had stage 3 CKD with one patient having stage 4 CKD. The indications for imaging were Rutherford category 1 ischemia in two patients and Rutherford category 3 or greater in the remaining five patients. Three patients underwent imaging due to critical limb ischemia with evidence of chronic tissue loss. Comorbidities and patient demographics are outlined in (Table 1).
| Variable | |
|---|---|
| Age | 72 (64,92) |
| Male Sex | 7/7 |
| Race | |
| White | 6/7 |
| Black | 0/7 |
| Asian | 1/7 |
| Hypertension | 7/7 |
| Coronary artery disease | 5/7 |
| Diabetes mellitus | 5/7 |
| Hyperlipidemia | 7/7 |
| Chronic kidney disease | 7/7 |
| Patient demographics are included for each patient undergoing feraheme-enhanced MRA and digital subtraction angiography. | |
Table 1: Patient demographics (N=7).
Pre-ferumoxytol serum creatinine did not significantly differ from post-ferumoxytol serum creatinine obtained after magnetic resonance imaging (1.81 ± 0.75 vs. 1.88 ± 0.75, p= 0.41). Similarly, GFR showed no significant change after Fe-MRA (40.9 ± 11.05 vs. 39.3 ± 11.78, P=0.47). Post-angiography serum creatinine and GFR did not differ significantly from pre-operative values with mean ± SD of 1.67 ± 0.56 (p=0.55) and 44.4 ± 12.03 (p=0.43), respectively. Iodinated contrast volumes used during selectiveangiography were significantly lower than volumes recorded for disease-matched control patients who did not undergo preoperative Fe-MRI, and thus underwent conventional aortogram with runoff (8240 ± 5206 vs. 29320 ± 15605, P<0.001). Following ferumoxytol administration, there were no acute allergic reactions and no episodes of anaphylaxis.
Following Fe-MRA and DSA, 3/7 patients underwent superficial femoral artery stenting following angiography and 1/7 patients underwent balloon angioplasty of the tibioperoneal trunk. The remaining 3/7 patients underwent only diagnostic angiograms, one of whom subsequently required an open femoral patch angioplasty.
Quality analysis performed for each Fe-MRA and DSA study revealed similar mean scores at the femoral, popliteal and tibial levels as demonstrated in (Table 3). There was generally a trend toward higher quality scores for DSA, but no difference met statistical significance at any level below the iliac arteries. Imaging at the iliac level proved difficult to compare given a large number of missing data points for DSA. Because the subtraction angiograms were performed selectively with a focus on the area of disease identified on Fe-MRA, incomplete imaging of the iliac level occurred in 10 patients, leaving only 4 for quality analysis. The mean iliac level quality score for DSA was 2.28 ± 0.91 as compared with a mean score of 3.57 ± 0.76 for Fe-MRI (p<0.0023).
| Arterial Level | Fe-MRA | DSA | p-value |
|---|---|---|---|
| Iliac | 3.57 (0.75) | 2.0 (0.82) | 0.002 |
| Femoral | 3.50 (0.56) | 3.67 (0.65) | 0.557 |
| Popliteal | 3.64 (0.74) | 4.0 (0) | 0.169 |
| Tibial | 3.93 (0.27) | 4.0 (0) | 0.463 |
| Fe-MRA=Feraheme-enhanced magnetic resonance imaging, DSA=digital subtraction angiography. | |||
Table 3: Qualitative analysis of patients undergoing both ferahemeenhanced MRA and digital subtraction angiography (N=7).
Regarding the degree of stenosis analysis, there was no statistically significant difference between estimates based on Fe-MRA vs. DSA. Differences in the estimate for the degree of stenosis were only evident for four patients. Two patients presenting with disease at the iliac level had the degree of stenosis underestimated on Fe-MRA, while the other two patients presenting with femoral level disease were found to have an exaggerated degree of stenosis on Fe-MRA in comparison with DSA. The management plan was not altered by the differences found at the time of contrast angiography for any patient.
Discussion
USPIO particle contrast agents have gained increasing popularity as an alternative to gadolinium based contrast agents for magnetic resonance imaging due to their established safety profile in the setting of CKD [7,13,14] and long intravascular halflife allowing both first-pass arterial imaging as well as blood-pool, or delayed imaging [15]. The clinical applicability of ferumoxytol as an off-label contrast agent has been demonstrated in small studies for a number of anatomic regions of interest including abdominal, intracranial, pulmonary, coronary and peripheral venous and arterial vasculature [8,11,15]. USPIO-enhanced MRA has shown particular promise in vascular surgery populations undergoing upper extremity dialysis access surveillance [16], post-endovascular aneurysm repair imaging [17,18], and imaging for peripheral arterial occlusive disease [9,12].
Our pilot evaluation of ferumoxytol as a contrast agent for preoperative planning in the setting of peripheral arterial occlusive disease demonstrates that Fe-MRA provides image quality that is comparable to the gold standard DSA. However, there were statistically significant differences with respect to estimation of the degree of stenosis on Fe-MRA and the degree of stenosis found on DSA. There were no documented adverse events associated with ferumoxytol administration and no significant alteration in renal function as estimated by serum creatinine and GFR. Pre-operative integration of Fe-MRA in patients with advanced CKD identified the region of disease in all patients allowing selective angiography to be performed with significantly reduced iodinated contrast loads.
In a recent report by Walker et al. [12], five patients imaged with Fe-MRA were compared with a control population of five patients who underwent MRA with gadolinium-based contrast. They demonstrated that image quality was comparable to gadoliniumbased studies and that the studies were adequate for operative planning. Their data also suggested that Fe-MRA may be superior for imaging the tibial level. The primary drawbacks from this report were the small sample size, unpaired comparison of the two groups and lack of comparison with the gold standard, DSA. They reported no adverse reactions or negative impact on renal function associated with Fe-MRA. Further analysis of Fe-MRA in the peripheral arterial vasculature is limited; however, Li et al. [9] did include peripheral arterial imaging of four patients in a feasibility study which similarly showed comparable image quality to gadolinium-based MRA with a reasonable safety profile.
Although the present report is the first to provide a valuable comparison between Fe-MRA and DSA, it does have a number of limitations including the small sample size and single institution nature of the study. The qualitative analysis used to compare different imaging modalities is subjective in nature, but inclusion of a quantifiable parameter in the degree of stenosis adds objectivity to our methodology. Furthermore, qualitative comparison is a common descriptive method reported in similar radiographic imaging studies [12,16].
Despite its significant advantages, ferumoxytol also has several drawbacks as a contrast agent. One of the distinguishing features of ferumoxytol is its high r1 and r2 relaxivity in comparison with gadolinium-based agents. This characteristic leads to significant susceptibility effect and potential signal loss on T1 imaging when administered at high concentrations [11]. Fananapazir et al. [19] reported on 61 abdominal MRI performed with ferumoxytol (3 mg/kg diluted to a total volume of 30 mL) bolus injected at 2 mL/sec. In this study, the incidence of vascular artifact mimicking thrombosis was 49% (30/61) which they attributed to susceptibility effects from concentrated ferumoxytol during the arterial phase. The concentration of ferumoxytol administered was significantly higher among the patients with vascular artifact on MRA, further supporting their claim. Though no thrombotic artifacts were recognized in our study focusing on peripheral arterial vasculature, the susceptibility effects associated with ferumoxytol during bolus administration must be taken into account, particularly when imaging the abdominal aorta or portal system. Though ideal MR protocols with ferumoxytol have not been established, a shorter echo time has been proposed as a means for reducing susceptibility artifact [20].
Recently, the FDA administered a safety announcement stating that they are strengthening the existing warning regarding serious, potentially fatal allergic reactions may occur with ferumoxytol administration. In clinical studies assessing the safety of ferumoxytol serious hypersensitivity reactions were reported in 0.2% of subjects, thus the original recommendation was that ferumoxytol is contraindicated for any patients with known hypersensitivity to ferumoxytol or any of its components. They have since added the contraindication of patients who have had any allergic reaction to an iron product. The FDA has also recommended that the drug be avoided in patients with multiple drug allergies as this population appears to be at higher risk of a serious adverse event. Due to these recent finding, further study of ferumoxytol as an alternative MR contrast agent in the setting of chronic kidney disease should proceed with caution.
Conclusion
We have demonstrated that Fe-MRA is a feasible imaging modality for operative planning in the setting of peripheral arterial disease with image quality and estimation of degree of stenosis that compare favorably with the gold-standard, DSA. There were no adverse events in the population of patients with advanced renal disease, but the recent FDA safety alert regarding ferumoxytol must be taken into account when determining the further use of this medication for imaging purposes. Appropriate safety measures should be firmly adhered to.
References
- Garimella PS, Hirsch AT (2014) Peripheral artery disease and chronic kidney disease: clinical synergy to improve outcomes. Adv Chronic Kidney Dis 21:460-471.
- Pasqualini L, Schillaci G, Pirro M, Vaudo G, Siepi D, et al. (2007) Renal dysfunction predicts long-term mortality in patients with lower extremity arterial disease. J Intern Med262:668-677.
- Paraskevas KI, Giannoukas AD, Mikhailidis DP (2009) Renal function impairment in peripheral arterial disease: an important parameter that should not be neglected. Ann VascSurg23:690-699.
- Rashid ST, Salman M, Myint F, Baker DM, Agarwal S, et al. (2004) Prevention of contrast-induced nephropathy in vascular patients undergoing angiography: a randomized controlled trial of intravenous N-acetylcysteine. J VascSurg40:1136-1141.
- Swaminathan S, Shah SV (2007) New insights into nephrogenic systemic fibrosis. J Am SocNephrol18:2636-2643.
- Prince MR, Zhang HL, Chabra SG, Jacobs P, Wang Y (2003) A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J XraySciTechnol11:231-240.
- Neuwelt EA, Hamilton BE, Varallyay CG, Rooney WR, Edelman RD, et al. (2009) Ultrasmallsuperparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int75:465-474.
- Stabi KL, Bendz LM (2011) Ferumoxytol use as an intravenous contrast agent for magnetic resonance angiography. Ann Pharmacother45:1571-1575.
- Li W, Tutton S, Vu AT, Pierchala L, Li BS, et al. (2005) First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmallsuperparamagnetic iron oxide (USPIO)-based blood pool agent. J MagnReson Imaging 21:46-52.
- Feraheme® (ferumoxytol) Injection for Intravenous (IV) Use.
- Bashir MR, Bhatti L, Marin D, Nelson RC (2015) Emerging applications for ferumoxytol as a contrast agent in MRI. J MagnReson Imaging 41:884-898.
- Walker JP, Nosova E, Sigovan M, Rapp J, Grenon MS, et al. (2015) Ferumoxytol-enhanced magnetic resonance angiography is a feasible method for the clinical evaluation of lower extremity arterial disease. Ann VascSurg 29:63-68.
- Bernd H, De Kerviler E, Gaillard S, Bonnemain B (2009) Safety and tolerability of ultrasmall superparamagnetic iron oxide contrast agent: comprehensive analysis of a clinical development program. Invest Radiol 44:336-342.
- Singh A, Patel T, Hertel J, Bernardo M, Kausz A, et al. (2008) Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis52:907-915.
- Hope MD, Hope TA, Zhu C, Faraji F, Haraldsson H, et al. (2015) Vascular Imaging With Ferumoxytol as a Contrast Agent. AJR Am J Roentgenol23:W1-W8.
- Sigovan M, Gasper W, Alley HF, Owens CD, Saloner D (2012) USPIO-enhanced MR angiography of arteriovenous fistulas in patients with renal failure. Radiology265:584-590.
- Ersoy H, Jacobs P, Kent CK, Prince MR (2004) Blood pool MR angiography of aortic stent-graft endoleak. AJR Am J Roentgenol182:1181-1186.
- Ichihashi S, Marugami N, Tanaka T, Iwakoshi S, Kurumatani N, et al. (2013) Preliminary experience with superparamagnetic iron oxide-enhanced dynamic magnetic resonance imaging and comparison with contrast-enhanced computed tomography in endoleak detection after endovascular aneurysm repair. J VascSurg 58:66-72.
- Fananapazir G, Marin D, Suhocki PV, Kim CY, Bashir MR (2014) Vascular artifact mimicking thrombosis on MR imaging using ferumoxytol as a contrast agent in abdominal vascular assessment. J VascIntervRadiol25:969-976.
- Neimatallah MA, Chenevert TL, Carlos RC, Londy FJ, Dong Q, et al. (2000) Subclavian MR arteriography: reduction of susceptibility artifact with short echo time and dilute gadopentetatedimeglumine. Radiology217:581-586.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences