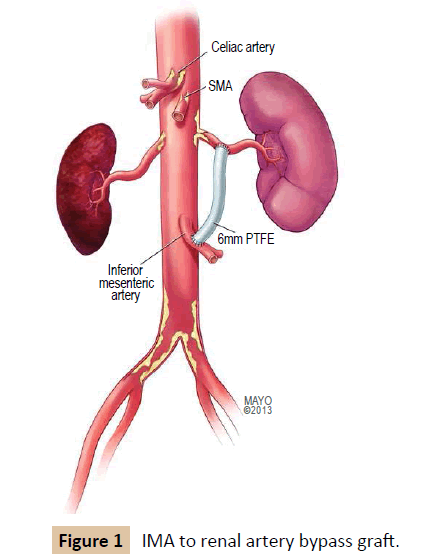
Inferior Mesenteric Artery as an Inflow for Unilateral Renal Artery Revascularization
Tariq Almerey, Daniel Kim, January Moore, Albert G Hakaim and Houssam Farres
DOI10.21767/2573-4482.100053
Tariq Almerey*, Daniel Kim, January Moore, Albert G Hakaim and Houssam Farres
Department of Surgery, Mayo Clinic's Campus in Florida, Surgery, FL 32224, USA
- *Corresponding Author:
- Tariq Almerey
Department of Surgery, Mayo Clinic's Campus in Florida
4500 San Pablo Rd S, Jacksonville
FL 32224, USA
Tel: 2056434311
E-mail: almerey.tariq@mayo.edu
Received date: June 14, 2017; Accepted date: July 04, 2017; Published date: July 13, 2017
Citation: Almerey T, Kim D, Moore J, Hakaim AG , Farres H (2017) Inferior Mesenteric Artery as an Inflow for Unilateral Renal Artery Revascularization. J Vasc Endovasc Surg. Vol. 2 No. 3:21. doi: 10.21767/2573-4482.100053
Abstract
Introduction: To report a successful unilateral renal revascularization via an inferior mesenteric artery to left renal artery bypass with return of renal function to baseline postoperatively.
Methods: A 73-year-old female presented with rapidly deteriorating renal function and uncontrolled hypertension secondary to ischemic nephropathy due to chronic renovascular occlusive disease. The patient had failed prior Percutaneous Transluminal Angioplasty (PTA) attempts. Preoperative evaluation showed the right kidney to be atrophied and normal size left kidney. Intraoperative exploration revealed porcelain abdominal aorta and both common and external iliac arteries were heavily calcified and were not suitable for appropriate clamping or to be used as an inflow for renal revascularization. Although the patient was asymptomatic, both celiac artery and Superior Mesenteric Artery (SMA) were nearly occluded and severely stenosed, respectively, at their origins. Therefore, the splenic artery and SMA were not used for reconstruction. Further exploration revealed a minimally diseased and widely patent Inferior Mesenteric Artery (IMA). A unilateral renal revascularization was performed via an IMA to left renal artery bypass using PTFE graft via a midline laparotomy.
Results: The patient was discharged on postoperative day 8 in good condition. Glomerular Filtration Rate (GFR) and blood pressure returned to baseline within 6 weeks postoperatively. CT angiogram undertaken 24 months postoperatively revealed a patent renal bypass. The patient maintained normal renal function and adequately controlled blood pressure 24 months postoperatively.
Conclusion: Open surgical correction of atherosclerotic renovascular disease can retrieve renal function in selected hypertensive patients with ischemic nephropathy. In the absence of other suitable inflow sources, the inferior mesenteric artery can be used as an alternative with acceptable outcomes.
Introduction
Renal Artery Stenosis (RAS) is a common condition that occurs in up to 5% of people with hypertension, and up to 7% of people 65 years or older [1-3]. Endovascular, medical therapy and surgery have been described for renal artery revascularization [4]. The aorta and iliac arteries are the most common used inflow source for conventional surgical revascularization. Other inflow sources such as the Gastroduodenal Artery (GDA), hepatic artery, splenic artery and Superior Mesenteric Artery (SMA) have been previously described. This is a report of an alternative inflow for renal artery revascularization using the Inferior Mesenteric Artery (IMA). Patient consent for publication was obtained.
Case Report
A 73-year-old female with a past surgical history significant for bilateral iliac arteries stenting and a past medical history significant for peripheral artery disease, uncontrolled hypertension, tobacco abuse, and hyperlipidemia presented with acute deterioration of her renal function. Glomular Filtration Rate (GFR) and creatinine at time of presentation to clinic was 12.8 and 3.0 respectively. She had normal renal function in the past 4 months. Renal ultrasound duplex exhibited elevated peak systolic velocities and chronic atrophy of the right kidney (right kidney: Resistive Index (RI) 0.4, kidney length 8 cm. Left kidney: RI 0.6, left kidney length 11.7 cm). Therefore, she was deemed a candidate for open left renal artery revascularization. Aortogram revealed bilateral critical renal artery stenosis due to significantly calcified atherosclerotic plaques at the renal artery orifices. Multiple attempts were made to cross the bulky plaque without success.
Both renal arteries were explored via a midline incision. Intraoperative findings were significant for circumferentially heavily calcified abdominal aorta and bilateral common and external iliac arteries. Therefore, they were not suitable for clamping or constructing an anastomosis. The celiac, superior mesenteric artery and splenic artery were exposed. All three appeared to be heavily calcified with several areas of significant stenosis and deemed not suitable candidates to be used as inflow. During dissection, the inferior mesenteric artery appeared to be of good caliber, without significant stenosis at its origin, and was deemed adequate for the site of the proximal anastomosis. The left renal artery measured 2.5-3.0 mm and appeared suitable for constructing an anastomosis. The right renal artery was heavily calcified and diminutive distal to its origin. In addition, the right kidney appeared atrophied. Therefore, the decision was made to create a bypass to the left renal artery only. No suitable vein was available as a conduit. Therefore, a 6 mm PTFE graft was used. The PTFE graft was passed behind the left renal vein, then anterolateral to the aorta. After intravenous administration of 5000 units of heparin, proximal and distal control of the IMA was obtained, a 1 cm arteriotomy was performed along the anterolateral aspect of the IMA. The graft was spatulated and anastamosed in an end-to-side fashion with 6-0 prolene. The PTFE graft was anastomosed to the left renal artery in similar end-to-side fashion to a 1 cm arteriotomy along the artery (Figure 1). After completion, intraoperative Doppler revealed excellent renal artery signals, and the graft demonstrated no kinks or twists to limit the flow. The colon was examined carefully and found to remain viable with adequate flow along its mesentery and the anti-mesenteric border. Once adequate hemostasis was achieved, the retroperitoneum was closed over the graft with 2-0 vicryl.
The patient was discharged on postoperative day 8 without complications. Glomerular filtration rate and blood pressure returned to baseline within 6 weeks postoperatively. CT angiogram undertaken 24 months postoperatively demonstrated a patent renal bypass graft. The patient maintained normal renal function and adequately controlled blood pressure 24 months postoperatively.
Discussion and Conclusion
Primary diseases of the renal arteries most commonly involve the large renal arteries, whereas secondary diseases involve intra-renal vessels. The two most common primary diseases of the renal arteries are atherosclerosis and fibromuscular dysplasia, which are associated with hypertension and ischemic nephropathy [4-8]. Atherosclerosis accounts for 90% of cases of Renal Artery Stenosis (RAS) and usually involves the ostium and proximal third of the main renal artery [6,7]. Fibromuscular dysplasia accounts for less than 10% of cases and usually involves the media and distal two thirds of the renal artery [8].
Medical therapy, endovascular and open revascularization are described and compared in the literature for the treatment of RAS. Several studies have demonstrated negligible difference between endovascular revascularization when compared with the best available medical therapy in atherosclerotic renal artery stenosis [4,9]. Other studies show that endovascular revascularization is effective in high-risk patients, such as those with acutely deteriorating renal function and uncontrolled hypertension [10,11]. Other authors showed that endovascular revascularization in addition to medical therapy lowered requirements for antihypertensive medications without an improvement in kidney function compared to medical therapy alone [12,13].
Abela et al. [12] compared surgical versus endovascular revascularization in atherosclerotic renal artery stenosis, and demonstrated better control of hypertension and a greater improve in renal function in the open intervention arm versus endovascular arm.
Medical and endovascular treatment have failed in this patient, so a surgical revascularization was performed. When an open revascularization approach is warranted, multiple options for revascularization are described, aorta and iliac artery being most commonly used as inflow sources [14,15]. In this case study, the aorta, iliac arteries, splenic artery, hepatic artery, and superior mesenteric artery were severely diseased. The inferior mesenteric artery appeared to be of good caliber and relatively little evidence of calcification. The patient tolerated the procedure well and renal function returned to baseline within 6 weeks. Renal function and blood pressure remained normal and adequately controlled after 24 months of follow up.
In conclusion, IMA to renal artery bypass is feasible and can be an alternative inflow source for revascularization with acceptable long term outcomes.
References
- Derkx FH, Schalekamp MA (1994) Renal artery stenosis and hypertension. Lancet 344: 237-239.
- Ram CV (1997) Renovascular hypertension. Curr Opin Nephrol Hypertens 6: 575-579.
- Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, et al. (2002) Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 36: 443-451.
- Riaz IB, Husnain M, Riaz H, Asawaeer M, Bilal J, et al. (2014) Meta-analysis of Revascularization Versus Medical Therapy for Atherosclerotic Renal Artery Stenosis. Am J Cardiol 114: 1116-1123.
- Lao D, Parasher P, Cho K, Yeghiazarians Y (2011) Atherosclerotic renal artery stenosis-diagnosis and treatment. Mayo Clin Proc 86: 649-657.
- Kaatee R, Beek FJ, Verschuyl EJ, vd Ven PJ, Beutler JJ, et al. (1996) Atherosclerotic renal artery stenosis: ostial or truncal? Radiology 199: 637-640.
- Dieter R, Schmidt W, Pacanowski J, Jaff MR (2005) Renovascular hypertension. Expert Rev Cardiovasc Ther 3: 413-422.
- Safian R, Textor S (2001) Renal-artery stenosis. N Engl J Med 344: 431-442.
- Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, et al. (2014) Stenting and Medical Therapy for Atherosclerotic Renal-Artery Stenosis. N Engl J Med 370: 13-22.
- Martinelli O, Malaj A, Antignani PL, Frati G, Belli C, et al. (2015) Renal stenting for kidney salvage in the management of renal artery atherosclerotic stenosis. Angiology 66: 785-791.
- Choi SS (2014) Atherosclerotic renal artery stenosis and revascularization. Cardiovasc Ther 12: 1419-1425.
- Abela R, Ivanova S, Lidder S, Morris R, Hamilton G (2009) An analysis comparing open surgical and endovascular treatment of atherosclerotic renal artery stenosis. EurJ Vasc Endovasc Surg 38: 666-675.
- Kumbhani DJ, Barvry AA, Harvey JE, de Souza R, Scarpioni R, et al. (2011) Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: A meta-analysis of randomized controlled trials. Am Heart J 161: 622-630.
- Libertino JA, Zinman L (1980) Renal revascularization using aortorenal saphenous vein bypass grafting. Surg Clin North Am 60: 487-501.
- Novick AC, Banowski LH (1979) Iliorenal saphenous vein bypass: an alternative for renal revascularization in patients with a surgically difficult aorta. J Urol 122: 243-245.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences