Initial Experience with Analogues of Prostaglandins for Haemodialysis Access-Induced Distal Ischemia
Marco Franchin, Gabriele Piffaretti, Patrizio Castelli and Matteo Tozzi
DOI10.21767/2573-4482.100018
Marco Franchin1,2,*, Gabriele Piffaretti1, Patrizio Castelli1 and Matteo Tozzi1
1Department of Surgery and Morphological Sciences, University of Insubria School of Medicine Circolo University Teaching Hospital, Varese, Italy
2Cardiothoracic and Vascular Department, Santa Maria Hospital, Terni, Italy
- *Corresponding Author:
- Marco Franchin
Vascular Surgery, Department of Surgery and
Morphological Sciences, Circolo University Hospital,
University of Insubria School of Medicine,
Via Guicciardini, 9 21100 Varese, Italy
Tel: +39-0332-393/259
Fax: +39-0332-278/581
E-mail: marco.franchin@hotmail.it
Received Date: June 13, 2016; Accepted Date: July 27, 2016; Published Date: August 03, 2016
Citation: Franchin M, Piffaretti G, Castelli P, et al. Initial Experience with Analogues of Prostaglandins for Haemodialysis Access- Induced Distal Ischemia. J Vasc Endovasc Surg. 2016, 1:3. doi: 10.21767/2573-4482.100018
Abstract
Introduction: Haemodialysis access-induced distal ischemia (HAIDI) is a common disabling complication that can be ascribed to an excess of blood flow through a haemodialysis vascular access, to the lack of arterial adaptation to the fistula and/or to arterial stenoses. Aim of the present study is to describe a preliminary experience with two analogues of prostaglandins, iloprost and misoprostol, for HAIDI treatment.
Materials and methods: Patients with HAIDI stage 1 and 2a were treated for 10 weeks with misoprostol 400 μg once per day. Patients with HAIDI stage 2b and 3 received a selective intra-arterial injection of iloprost 0.025 mg and subsequently an intravenous continuous injection of iloprost 1.25 ng/kg/min for 72 h, finally misoprostol 400 μg once per day for 10 weeks. Evaluation of treatment benefit included vascular fluorescence quality control, pain evaluation and QOL assessment.
Results: Sixteen patients were treated with PG: twelve with HAIDI stage 1 and 2a, four with HAIDI stage 2b and 3, major local or systemic morbidity and adverse effects to the drugs were not observed. In all cases symptoms, QOL and vascular fluorescence improvement was observed. At the end of the study 25% of the patients did not present HAIDI symptoms while 75% negligible symptoms. All the patients refused surgical option.
Conclusion: The treatment of HAIDI has demonstrated to be safe and effective in a selected cohort of patients
Keywords
Prostaglandins; Ischemia; Haemodialysis; Fluorescence; angiography
Abbreviations
AU: arbitrary units; COPD: chronic obstructive pulmonary disease; CVD: cerebrovascular disease; HD: haemodialysis; HAIDI: haemodialysis access-induced distal ischemia; ICG: indocyanine green; IHD: ischemic heart disease; MVQOLI: 15-Missoula-VITAS quality of life index-15; PG: prostaglandin; QoL: quality of life; ROI: region of interest; SD: standard deviation; TX: thromboxane
Introduction
Haemodialysis access-induced distal ischemia (HAIDI) is commonly described after vascular access placement and can be attribute to the concern of blood flow restriction, the excess of blood flow through the fistula and to the lack of arterial adaptation or collateral flow reserve [1]. Many authors underlined the role of reversed blood flow direction in forearm arteries [2,3] but this evidence has been downsized [4]. More recently, literature has stressed the role of loco regional hypotension resulting from pressure drop due to progressive arterial stiffening associate to arteriovenous anastomotic turbulence [2,3,5,6]. Up to 5% of dialysis patients require invasive treatment for severe HAIDI, usually 1-2 years after arteriovenous fistula creation [7]. Authors experience with analogue of prostaglandins (PG) for the treatment of lower limbs ischemia has addressed the attention on arteriolar peripheral district resistance and collateral pathway role in balancing ischemic situation. Aim of the present paper is to describe our Centre experience with PG in HIDI management. Iloprost and misoprostol were tested. They are two stable prostacyclin analogues, effective as vasodilator and inhibitor of the adhesion and activation of thrombocytes. Pharmacological therapy was limited to those cases where a single cause of hand ischemia was not identifiable and in particular when an inadequate collateral arterial network was documented.
Materials and Methods
Study design and settings
The present is a single centre experience with retrospective analysis of prospectively collected data in a twenty-four months period between January 2014 and December 2015.
Patient’s cohorts, exclusion criteria, diagnostic and decision algorithm
All the consecutive patients referred to our Department with persistent episodes of HAIDI were identified. HAIDI history was evaluated on the basis of the Vaes et al., four-stage classification [4]. All the patients received a physical examination and echocolor- Doppler evaluation. Additionally, a complete angiography assessment of arm arterial vasculature and arteriovenous fistula was reserved for patients with severe HAIDI (stage 2b or more).
Exclusion criteria were HAIDI stage 4 with tissue loss (ulceration and necrosis) with indication to immediate intervention to preserve hand function. Additionally, patient with critical arterial stenosis assessed with echo-color-Doppler or angiography were excluded.
Subsequently, patients were divided in two groups on the basis of clinical presentation of hand ischemia: Group 1) Mild-HAIDI stage 1 and 2a; Group 2) Severe-HAIDI stage 2b and 3.
Once excluded the presence of critical vascular stenoses affecting the upper arm arterial pathway, patients in group 1 received Misoprostol 400 μg once per day for 10 weeks. Otherwise, patients in group 2 during angiography received a selective intra-arterial injection of iloprost 0.025 mg and subsequently an intravenous continuous injection of iloprost 1.25 ng/kg/min for 72 h under close clinical control. Subsequently, patients in group 2 received Misoprostol 400 μg once per day for 10 weeks.
All the patients did not receive a central venous catheter and continued to undergo dialysis through the vascular access.
Evaluation and follow-up
Study duration was 10 weeks. Evaluation of treatment benefit included: vascular fluorescence quality control, pain evaluation and quality of life (QOL) assessment.
Vascular quality control
Vascular fluorescence image control was performed with Indocyanine green (ICG) at baseline, at the end of the fifth week and at the end of the study. ICG 5 mL at a concentration of 0.3 mg/mL/kg was injected intravenously. For ICG fluorescent imaging, we used a laparoscopic system (TELECAM SL II-Storz; Tuttlingen–D), a 10 mm laparoscope applicable for white light and ICG-imaging. The evaluation was conducted always in the same conditions: the patient was supine, with at least 15 min rest before examination and with the arm abducted, the room temperature was 22°C. We considered the hand as region of interest (ROI) for fluorescence study. Subsequently, we calculated the median fluorescence intensity (in arbitrary units-AU) into the ROI over the whole images series obtaining a dataset of binary values. The present protocol was validated in a previous paper published by the same authors [12]. In other papers on the same topic we found commonly employed flow measurement methods such as systolic blood pressure and plethysmography of the index finger [4]. First of all, we have to admit that we are unfamiliar with this technique. Secondarily, even more evidences sustain the efficacy of fluorescence study distal limb perfusion [8-10] in comparison to other non-invasive technique [11]. Finally, our interest was mainly addressed to the quality of hand perfusion more than flow evaluation.
Pain and QOL assessment
Patients received a questionnaire in diary form. In literature are reported several questionnaire for the assessment of HAIDI. In authors opinion the most complete appear to be the Vaes et al., form (4c). However, we preferred to use a more simple and patient-friendly form in order to reduce the patient’s distress. In fact, we asked to fill it at the end of every haemodialysis for the whole study period of 10 weeks. Patients had to record the numbers of DASS attack, duration, intensity, analgesic drugs and dose eventually necessary for pain management. A 10-point patient–completed scale was used to determine the severity of pain where 0 represented no pain and 10 a very severe pain. Patients started their diary at first examination (visit–1) before treatment.
The assessment of QOL was done employing the Missoula-VITAS (MV) QOL Index–15 Italian translation at baseline. MVQOLI-15 was administered at first examination, at the end of the fifth week and at the end of the study. MVQOLI-15 consisted of five dimensions: symptoms, functioning, interpersonal relationships, wellness, and spirituality. In each area, three types of information were registered: a) evaluation (subjective measurement of the actual state, 5-point scale from -2 to +2); b) satisfaction (degree of acceptance of the actual state, 9-point scale from -4 to +4); c) significance (degree of impact on the overall QOL, 5-point scale from 1 to 5). The score was calculated as follow: (evaluation + satisfaction) x significance [13].
Definitions
Definitions and outcomes criteria were defined accordingly to the Committee on Reporting Standards of the Society for Vascular Surgery and the American Association for Vascular Surgery (SVS/AAVS) on AV accesses [14]. Definition of HAIDI is quite controversial. Actually in literature we can find several different definition of this pathology. In particular it is common to indicate it as dialysis access-associated steal syndrome (DASS) or simply hand ischemia. In authors opinion those definition are all correct. Otherwise, in this context HAIDI appears to be haemodynamic centred and consequently appropriate.
Data analysis
Quantitative fluorescence image analysis was done with an open source program (ICY 1.7.3.0 for Linux) developed by the Quantitative Image Analysis Unit at Institute Pasteur, Paris. Data were collected and tabulated in a dedicated SQL database (Sqliteman 1.2.2 for Linux). Continuous variable are reported as median (range: minimum–maximum) while age as mean (± SD; range: minimum–maximum). For counts and categorical data, frequencies are reported with percentage in parentheses. Nonparametric Wilcoxon matched-pairs signed rank test was employed to compare the outcome of the two groups of patients. The statistical analysis was performed with PSPP 0.7.9 for Linux.
Results
Patients, hospitalization and follow-up
We identified 19 patients; there were 9 (47.4%) males. Overall, mean age was 60 years (± 15 years, range: 35-79). Patients were divided as follow: group 1 n=12 (63.2%), group 2 n=7 (36.8%). In group 2, completion angiography documented in 3 patients with auto genous vascular access a diffuse arterial stenoses and a very high-flow fistulas. In accordance with exclusion criteria, those 3 patients were omitted, and they underwent surgery: n=2 fistula proximalisation, n=1 arteriovenous anastomosis re-do. The remaining 16 (84.2%) patients were included in the study and treated with PG. Demographic data, co-morbidities and vascular access characteristics are summarized in Table 1. The two groups were well matched in terms of gender, age and comorbidities; in particular diabetes was present in 7 cases (group 1 n=4, group 2 n=3). In-hospital mortality was not observed; major local or systemic morbidity and adverse effects to the drugs were not observed. Median of hospitalization time was 78 h (range: 74-85 h). Patency of the vascular accesses was 100% at discharge. No patient was lost during the follow-up. Vascular access cannulation was performed regularly according to dialysis schedule. No patient received central venous catheter for haemodialysis. Table 2 reports results in detail.
| Variable | Patients (n=16) | |
|---|---|---|
| Demographic data | ||
| Female | 7 (58.3) | |
| Age, years | 60 ±15 | |
| Time of HD treatment, years | 6 | |
| Comorbidity | ||
| Smoking | 10 (62.5) | |
| IHD | 10 (62.5) | |
| Hypertension | 9 (56.3) | |
| Diabetes | 7 (43.8) | |
| COOPD | 6 (37.5) | |
| CVD | 2 (12.5) | |
| Type of vascular access | ||
| Autogenousvascular access | 9 (56.3) | |
| Middle forearm | 5 (31.3) | |
| Brachicephalic | 4 (25.0) | |
| Prosthetic vascular access | 7 (43.8) | |
| Radio - basilic | 4 (25.0) | |
| Brachial - basilic | 2 (12.5) | |
| Radial - cephalic | 1 (6.3) | |
| Group 1 | ||
| n | 12 (75.0) | |
| Female | 4 (25.0) | |
| Age (years ± SD) | 56 ± 7 | |
| Autogenousvascular access | 7 (43.8) | |
| Prosthetic vascular access | 5 (31.3) | |
| Group 2 | ||
| n | 4 (25.0) | |
| Female | 3 (18.8) | |
| Age (years ± SD) | 65 ± 8 | |
| Autogenousvascular access | 2 (12.5) | |
| Prosthetic vascular access | 2 (12.5) | |
Table 1: n: number; HD: haemodialysis; IHD: ischemic heart disease; COPD: chronic obstructive pulmonary disease; CVD: cerebrovascular disease. Continuous data are shown as the mean ± standard deviation or the mean (range) and categorical data as number (%).
| Time 0 | 5ft week | End of the study | p | ||||
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | ||
| HAIDI symptoms | |||||||
| Numer of attacks | 12 | 4 | 11 | 4 | 8 | 4 | 0.18 |
| Duration (sec) | 360 | 420 | 110 | 180 | 45 | 45 | 0.32 |
| Severity (1 - 10) | 7 | 9 | 4 | 6 | 1.5 | 2.5 | 0.18 |
| Vascular quality control | |||||||
| Fluorescence intensity (AU) | 23 | 20 | 35 | 30 | 39 | 33 | 0.11 |
| Latency of the peak (sec) | 35 | 44 | 24 | 30 | 21 | 24 | 0.18 |
| Quality of life | |||||||
| MVQoL Index - 15 | 18 | 13 | 22 | 21 | 26 | 24 | 0.18 |
Table 2: Results of the study. Sec: seconds; AU: arbitrary units; MVQoL: Missoula-VITAS quality of life.
Number of HAIDI attacks
At baseline all patients (100%) reported HAIDI symptoms during dialysis. In group 1 the number of patients with HAIDI symptom at the end of the tenth week was 8/12 (66.7%). In group 2 all patients (100%) reported HAIDI symptoms at the end of the study.
Vascular quality control
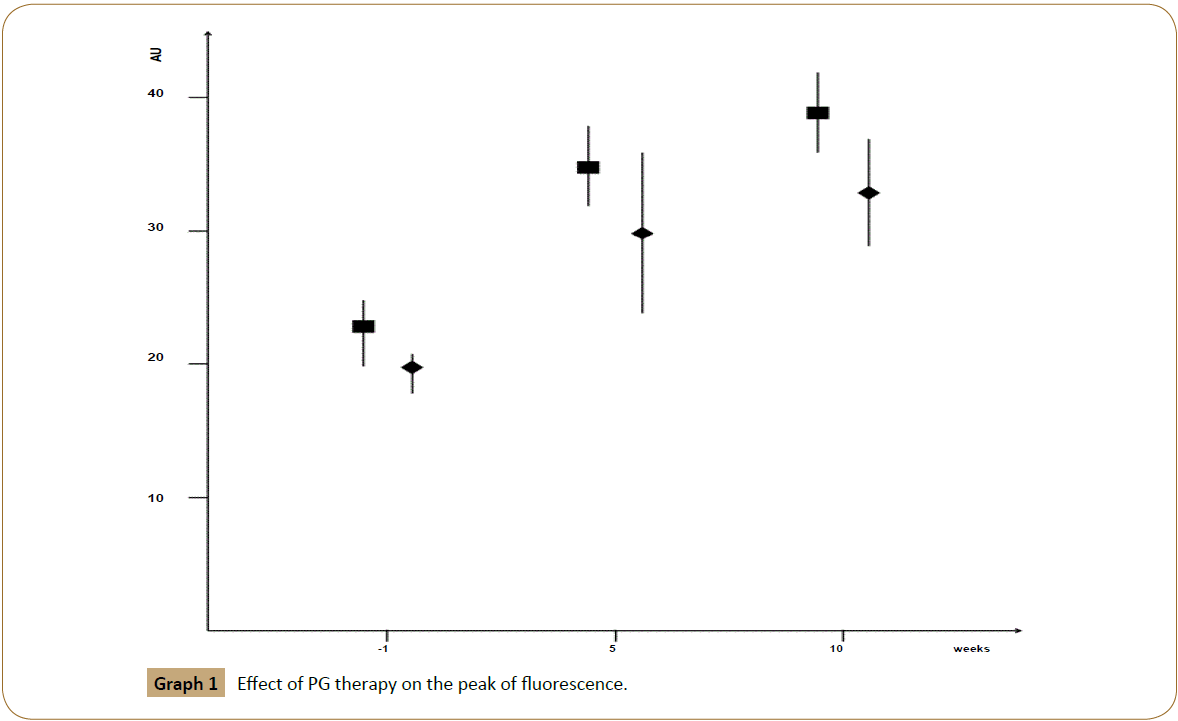
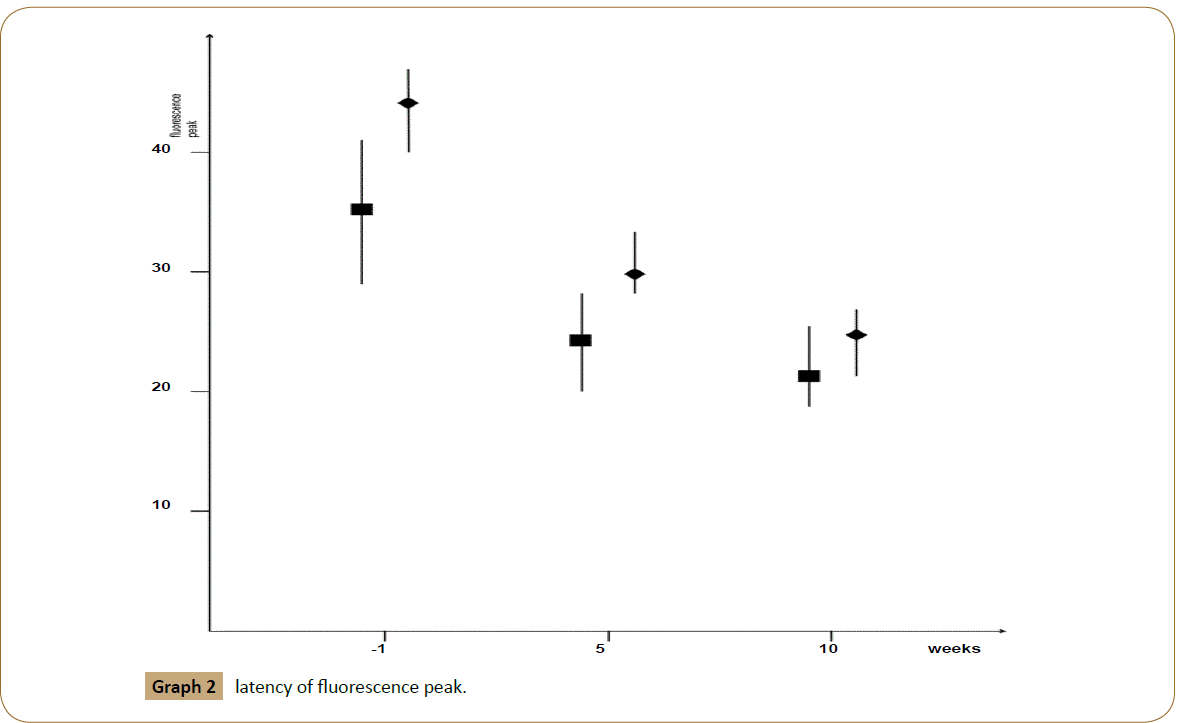
At baseline (visit-1) the median fluorescence intensity was 22 (range: 18-25) arbitrary units (AU): in group 1 the median fluorescence intensity was 23 (range 20-25) AU while in group 2 the median fluorescence intensity was 20 (range 18-21) AU. At the end of the fifth week the median fluorescence intensity was 31 (range: 24-38) AU: in group 1 the median fluorescence intensity was 35 (range 32 - 38) AU while in group 2 the median fluorescence intensity was 30 (range 24–36) AU. Finally, at the end of the study the median fluorescence intensity was 35 (range: 29-42) AU: in group 1 the median fluorescence intensity was 39 (range: 36-42) AU while in group 2 the median fluorescence intensity was 33 (range: 29-37) AU. Additionally, we noticed a difference in latency of fluorescence peak throughout the treatment. At baseline the median latency of fluorescence peak was 38 (range: 29-47) seconds: in group 1 the median latency was 35 (range: 29-41) sec while in group 2 the median latency was 44 (range: 40-47) sec. At the end of the fifth week the median latency of fluorescence peak was 26 (range: 20-33) sec: in group 1 was 24 (range 20-28) sec while in group 2 the median was 30 (range 28-33) sec. Finally, at the end of the study the median latency of fluorescence peak was 22 (range: 18-26) sec: in group 1 was 21 (range: 18-25) sec while in group 2 was 24 (range: 21-26) sec [Graphs 1 and 2].
Duration of HAIDI symptoms
The median duration of HAIDI attacks was 6 h (range: 4-7 h) in group 1 and 7 (range 4-9 h) h in group 2. The duration of symptoms decreased quickly after iloprost intravenous administration and during all the period of misoprostol administration. At the end of the study median duration of HAIDI attack was in both group 45 (range: 0-2 h) min.
Median severity of HAIDI symptoms
The median severity of HAIDI attacks was 7 (range: 6-8) in group 1 and 9 (range 9-10) in group 2. In both groups, the severity of symptoms decreased immediately after iloprost intravenous administration: at second day was 6 (range: 3-7) in group 1, 7 (range: 5-7) in group 2. For the subsequent 6 days it remained quite steady. After administration of misoprostole we noticed less evident but constant bettering of symptoms. At the end of the study median severity of HAIDI was 1.5 (range: 0-3) in group 1, 2.5 (range 2-3) in group 2.
Pain management
All patients (100%) needed analgesic drugs administration at baseline. In group 1, 7/12 (58.4%) patients received Paracetamol (median 2 g a day, range: 1-3 g) and 5/12 (41.6%) Paracetamol plus Codeine (median 2 g/120 mg a day, range 500 mg/30 mg-3 g/180 mg). At the end of the study only 1/12 (8.3%) patient received Paracetamol 1 g due to the persistence of pain. In group 2, 1/4 (25.0%) patients received Paracetamol 3 g a day, 2/4 (50.0%) Paracetamol 1 g plus Codeine 60 mg a day, 1/4 (25.0%) patient received Oxycodone 5 mg plus Paracetamol 325 mg a day. At the end of the study only 1/4 (25.0%) patient received Paracetamol 1 g due to the persistence of pain.
Quality of Life
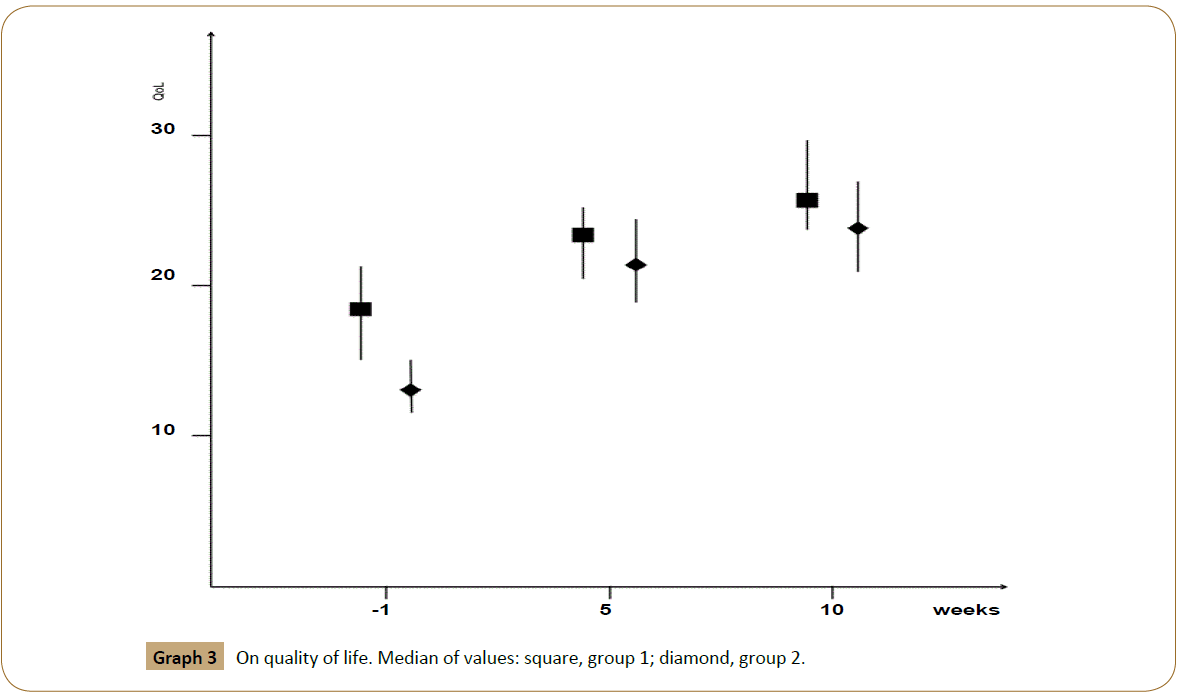
At baseline, the median total score of QoL was found to be 15 (in the range 0-30). The median score of QoL was higher in group 1 (value=18, range 15-21) if compared to group 2 (value=13, range 12-15). In both groups, the score of QoL increased rapidly during hospitalization after intravenous treatment with Iloprost. Therefore we noticed in both groups an improvement in QoL with a lower but continuous trend. Table 3 reports QoL results depending on the five dimensions MVQoL Index-15. At the end of the study QoL score was 26 (range 24-30) in group 1, 24 (range 21-27) in group 2. All the patients judged their QoL not bad and refused a definitive surgical treatment (graph 3).
| Time 0 | 5ft week | End of the study | ||||
|---|---|---|---|---|---|---|
| Dimensions | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 |
| Symptoms | -12 | -16 | -7 | -9 | 0 | -3 |
| Fnction | 15 | 14 | 14 | 14 | 10 | 12 |
| Relationships | 15 | 15 | 15 | 16 | 13 | 15 |
| Wellness | -8 | -8 | -6 | -8 | 0 | -5 |
| Spirituality | 8 | 8 | 6 | 8 | 3 | 5 |
| Total score | 18 | 13 | 22 | 21 | 26 | 24 |
Table 3: Quality of life (QOL) median results depending on the five dimensions of Missoula-VITAS QOL Index–15.
Discussion
HAIDI has become nowadays a growing problem [15]. Three main factors can create a symptomatic ischemic steal: 1) inadequate collateral arterial network; 2) a very high flow fistula; 3) a preexisting vascular occlusive disease. Generally, a combination of those three cause leads to a distal hypo perfusion ischemic syndrome [16]. Additionally, lower arm blood flow diminishment in presence of arteriovenous fistula can lead to critically reduced arterial pressures in the hand [2,17]. Generally, after the arteriovenous anastomosis creation, a decreased peripheral resistance lead to increased arterial flow with subsequent augmented wall shear stress inducing endothelial nitric oxide (NO) release. NO is fundamental for vasodilatation, inflow artery remodelling, and recruiting adjacent collaterals bypassing the anastomosis [18]. It is noteworthy that NO synthesis is diminished in diabetic and uremic patients due to endothelial dysfunction [19]. Additionally, diabetes and aging reduce the collateral pathway recruitment in reply to ischemia [20].
In our series, in two cases a severe arterial stenosis was documented and in one case a very high-flow fistula was found. In all those cases a radiological or surgical treatment solved symptoms. In the remaining 16 cases it was not possible to find just a single cause of the syndrome. We have to admit that diabetes and other comorbidities were well matched between the two groups. Consequently, and with regard to the small number of patients treated, it was not possible to find a significant correspondence among comorbidities, HAIDI onset and outcomes. Otherwise, a lack of arteriolar collaterals was always present with an image of diffuse “bare tree”. Subsequently, in author’s opinion HAIDI onset was mainly due to an inadequate collateral arterial network. Therefore, the study proposal was to employ PG to optimize arteriolar network bettering the distal vascularization in patients affected by HAIDI. Preliminary results are encouraging. In fact, the analysis of vascular fluorescence demonstrated that PG treatment gave an increasing of the median fluorescence intensity of 37.1% (group 1, 40.0%; group 2, 39.3%) in the whole group and a reduction in latency of fluorescence peak of 42.1% (group 1, 40.0%; group 2, 45.5%). Those results are in agreement with subjective pain feeling. In fact, accordingly with patients’ diary questionnaires, ischemic attack considerably decreased in both groups in terms of intensity (group 1, 78.6%; group 2, 72.2%) and duration (group 1, 87.0%; group 2, 89.3%). Unfortunately, all the patients in group 2 continued to present HAIDI at the end of the study while in group 1 only 8 (33.3%).
Nevertheless, QoL improved in both groups and none patient in group 2 consent to operative treatment for residue HAIDI. Admitting the small cohort of patients, results are not statistically influenced by severity of onset symptoms.
Generally, a spread range of different coexisting reason can contribute to determinate DASS. For example, diabetes and other co-morbidities commonly affecting uremic patients, including HD treatment, can produce diffuse arterial stenoses. Additionally, female patients with small arteries and highflow fistulas may exceed the capacity of the feeding arterial system even in absence of arterial inflow disease. In literature, no previous paper referring the use of PG for the treatment of HAIDI was found. The present study was based on author's experience in treatment of critical limb ischemia and Raynaud phenomenon with PG. In fact, clinical trials suggested the PG could reduce the symptoms of intermittent claudication [21]. It is notable that some authors proposed PG employment in addition to surgical treatment in patients with severe peripheral arterial disease or for limb salvage where revascularization was not possible [22]. Literature well documented the usefulness of PG for the treatment of RP in patients with systemic sclerosis [23]. PG and thromboxane (TX) are lipid signalling molecules active in an extensive variety of physiologic and pathological processes including gastric acid secretion, muscular contraction and haemostasis. PG (PGE2, PGD2, PGI2, PGF2) and TX (TXA2) bind distinct G-protein coupled receptors (G-PCRs: EP, DP, IP, FP, TP) modulating cyclic adenosine monophosphate and/or phosphor inositol turnover and consequently intracellular concentration of calcium [24]. Iloprost and misoprotol are stable analogue of PG. They bind respectively PGI2 receptors and PGE2 EP3-EP4- EP5 receptors. Both drugs increase adenylate cyclase activity and inhibit thrombine-induced Ca2+ mobilization [25] with the result of a long-duration vessel dilatation. In addition to artery dilatation PG are inhibitor of platelet adhesion and activation of thrombocytes [26] through the suppression of production of transforming growth factor-β, soluble adhesion molecule 1, soluble vascular adhesion molecule 1 and E-selectine [27,28]. In our experience we treated patients accordingly to the drug instruction for use. We did not report adverse effects generally described with PG such as hypokalaemia, hypotension, apnea/ bradycardia [29,30]. The FADOI 2bPILOT Study demonstrates the efficacy of intravenous 12 months iloprost treatment in addition to standard therapy in patients with peripheral artery disease. However, in their data discussion the authors underlined the difficult feasibility of a long intravenous therapy in terms of: 1) hospitalization settings, 2) challenge for health organization, 3) patients’ QOL [31]. For that reason they concluded that a more user-friendly and home-based way of administration of the drug was desirable. For that reason, authors of the present paper decided to reduce the dose and the duration of the intravenous treatment and consequently of the hospitalization. In contrast, an oral therapy was administered at home. It is imperative to emphasise the efforts encountered scheduling haemodialysis treatments for hospitalised patients. Additionally, the possibility to reduce the hospitalisation time could have positive benefit on treatment costs. Finally, a debate is still open in literature on the effective dose of PG [32]. Otherwise, a comparative randomized clinical trial did not find any difference in terms of efficiency of low-dose and high-dose iloprost for treatment of Raynaud's phenomenon. Different results were not obtained with different dose and therapy duration [33,34]. Traditionally, HAIDI treatment includes a wide variety of operative treatment: angioplasty, ligation of the fistula, distal revascularization with interval ligation, revision using distal inflow, banding, proximalization of arterial inflow, distal radial artery ligation [32,35,36]. From a clinical point of view, the evidence of a benefit in HAIDI treatment with PG gains a therapeutic relevance. In fact, it is too limiting to take in count only a surgical option against a multiple factorial pathophysiology. Larger and more prolonged studies should therefore be performed to establish any relationship between PG and peripheral vascular effects and favourable clinical outcomes in patients with HAIDI.
Study Limitations
Authors remind that present paper reports preliminary results with analogues of PG for HAIDI treatment. Nonetheless, it has several limitations: the cohort is small, the study is retrospective in nature, and that period considered is short. Moreover, the study design could be criticized. In fact, the experience lacks of comparison with another technique. We admit that the natural evolution of distal ischemia sometime could be favourable. Consequently, a comparison with a cohort of patients untreated should unequivocally demonstrate the efficacy of the therapy. However, as reported in table 1, patients enrolled had a long history of HD and the natural resolution of HAIDI was improbable. Nevertheless, future larger and longer-term studies are desirable to further evaluate the benefits of PG on HAIDI. Probably, they should unambiguously emphasis the effects on clinical outcome in comparison to placebo or surgical techniques.
Conclusion
Admitting the several limitations of present study we could prove a significant effect of PG treatment. PG might have a primary place in the therapeutic armamentarium in HAIDI treatment. In fact, in selected patients PG allows to optimize arterial pathway, minimizing symptoms. Both the vascular fluorescence assessment and the clinical evaluation confirmed the efficacy of the therapy reducing the necessity of a surgical treatment.
References
- Malik J, Tuka V, Kasalova Z, Chytilova E, Slavikova M, et al. (2008) Understanding the dialysis access steal syndrome. A review of the etiologies, diagnosis, prevention and treatment strategies. J Vasc Access 9:155-166.
- Anaya-Ayala JE, Pettigrew CD, Ismail N, Diez-De Sollano AL, Syed FA, et al. (2012) Management of dialysis access-associated “steal” syndrome with DRIL procedure: challenges and clinical outcomes. J Vasc Access 13: 299-304.
- Vaes RH, Scheltinga MR (2012)Side branch ligation for haemodialysis-access-induced distal ischaemia. Eur J VascEndovascSurg44:452-456.
- Vaes RH, Tordoir JH, Scheltinga MR (2013) Blood flow dynamics in patients with hemodialysis access-induced hand ischemia. J Vasc Surg58:446-451.
- Zanow J, Krueger U, Reddemann P, Scholz H (2008) Experimental study of hemodynamics in procedures to treat access-related ischemia.JVascSurg48:1559-1565.
- Scheltinga MR, Bruijninckx CM (2012)Haemodialysis access-induced distal ischemia (HAIDI) is caused by loco-regional hypotension but not by steal.Eur J VascEndovascSurg43:218-223.
- Scheltinga MR, van Hoek F, Bruijninckx CM (2009) Time of onset in hemodialysis access-induced distal ischemia (HAIDI) is related to the access type.Nephrol Dial Transplant24:3198-3204.
- Anghel EL, Falola RA, Kim PJ (2016) Fluorescence Technology for Point of Care Wound Management. Surg Technol Int.
- Guang H, Cai C, Zuo S, Cai W, Zhang J, et al. (2016) Multiparametric evaluation of hindlimb ischemia using time-series indocyanine green fluorescence imaging. J Biophotonics.
- Terasaki H, Inoue Y, Sugano N, Jibiki M, Kudo T, et al. (2013) A quantitative method for evaluating local perfusion using indocyanine green fluorescence imaging. Ann Vasc Surg27:1154-1161.
- Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, et al. (2008) Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbscomparison with other noninvasive diagnostic methods. J Vasc Surg47:318-323.
- Tozzi M, Boni L, Soldini G, Franchin M, Piffaretti G (2014) Vascular Fluorescence Imaging Control for Complex Renal Artery Aneurysm Repair Using Laparoscopic Nephrectomy and Autotransplantation. Case Reports in Transplantation.
- Theofilou P, Aroni A, Ralli M, Gouzou M, Zyga S (2013) Measuring health: related quality of life in hemodialysis patients. Psychometric properties of the Missoula-VITAS Quality of Life Index (MVQOLI-15) in Greece. Health Psychol Res1:e17.
- Sidwy AN, Spergel LM, Besarab A, Allon M, Jennings WC, et al. (2008) The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg48:2S-25S.
- Beathard GA, Spergel LM (2013) Hand ischemia associated with dialysis vascular access: an individualized access flow-based approach to therapy. Semin Dial 26:287-314.
- Leon C, Asif A (2007) Arteriovenous access and hand pain: the distal hypoperfusion ischemic syndrome. Clin J Am Soc Nephrol2:175-183.
- Vaes RH, Wouda R, Teijink JA, Scheltinga MR (2015) Venous Side Branch Ligation as a First Step Treatment for Haemodialysis Access Induced Hand Ischaemia: Effects on Access Flow Volume and Digital Perfusion. Eur J Vasc Endovasc Surg50:810-814.
- Achneck HE, Sileshi B, Li M, Partington EJ, Peterson DA, et al.(2010) Surgical aspects and biological considerations of arteriovenous fistula placement.Semin Dial 23:25-33.
- van Golde JM, Ruiter MS, Schaper NC, Voo S, Waltenberger J, et al.(2008) Impaired collateral recruitment and outward remodellingin experimental diabetes.Diabetes57:2818-2823.
- Semenza GL (2010) Vascular responses to hypoxia and ischemia. ArteriosclerThrombVascBiol30: 648-652.
- Robertson L, Andreas A (2013) Prostanoids for intermittent claudication. Cochrane Database Syst Rev.
- Vitalle V, Monami M, Mannucci E (2016) Prostanoids in patients with peripheral arterial disease: A meta–analysis of placebo-controlled randomized clinical trial. J Diabetes Complications 30:161-166.
- Garcìa de la PLP, Nishishinya MB, Pereda CA, Loza E, Sifuentes Giraldo WA, et al. (2015) Efficacy of Raynaud's phenomenon and digital ulcer pharmacological treatment in systemic sclerosis patients: a systemic literature review. Rheumatol Int 35:1447-1459.
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD (2001) Prostanoi receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol41:661-690.
- Oka M, Negishi M, Yamamoto T, Satoh K, Hirohashi T, et al. (1994) Prostacyclin (PGI) receptor binding and cyclic AMP synthesis activities of PGI1 analogues, SM-10906 and its methyl ester, SM-10902, in mastocytoma P-815 cells. Biol Pharm Bull17:74-77.
- Stratton R, Shiwen X, Martini G, Holmes A, Leask A, et al. (2001) Iiloprost suppresses connective tissue growth factor production in fibroblasts and in the skin of scleroderma patients. J Clin Invest108:241-250.
- Bruns M, Herrmann K, Haustein UF (1994) Immunologic parameters in systemic sclerosis. Int J Dermatol 33:25-32.
- Denton CP, Bickerstaff MC, Shiwen X, Carulli MT, Haskard DO, et al. (1995) Serial circulating adhesion molecule levels reflect disease severity in systemic sclerosis. Br J Rheumatol 34:1048-1054.
- Alhussin W, Verklan MT (2016) Complications of Long–Term Prostaglandin E1 Use in Newborns With Ductal–Dependent Critical Congenital Heart Disease. J Perinat Neonatal Nurs 30:73-79.
- O'Connell C, Amar D, Boucly A, Savale L, Jais X, et al. (2016) Comparative Safety and Tollerance of Prostacyclins in Pulmonary Hypertension. Drug Saf.
- Mazzone A, Di Salvo M, Mazzucca S, Valerio A, Gussoni G, et al. (2013) Effects of iloprost on pain-free walking distance and clinical outcome in patients with severe stage IIb peripheral arterial disease: the FADOI 2bPILOT Study. Eur J Clin Invest43:1163-1170.
- Tozzi M, Franchin M, Ietto G, Soldini G, Chiappa C, et al. (2014) A modified stapling technique for the repair of an aneurysmal autogenous arteriovenous fistula. J Vasc Surg 60:1019-1023.
- Bali G, Schwantzer G, Aberer F, Kraenke B, Aberer E (2011) Discontinuing long–term Iloprost treatment for Raynaud's Phenomenon and systemic sclerosis: a single–center, randomized, placebo-controlled, double-blind study. Acta Dermatovenerol Alp Pannonica Adrat20:13-21.
- Kawald A, Burmester GR, Hurscher D, Sunderkotter C, Riemekasten G (2008) Low versus high-dose iloprost therapy over 21 days in patients with secondary Raynaud's phenomenon and systemic sclerosis: a randomized, open, single-center study. J Rheumatol 35:1830-1837.
- Leake AE, Winger DG, Leers SA, Gupta N, Dillavou ED (2015) Management and outcomes of dialysis access-associated steal syndrome. J Vasc Surg61:754-760.
- Tozzi M, Franchin M, Ietto G, Soldini G, Carcano G, et al. (2014) Initial experience with the Gore Acuseal graft for prosthetic vascular access. J Vasc Access15:385-390.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences