Outcomes of Percutaneous-First Femoral Artery Access for Fenestrated and Branched Endovascular Aneurysm Repair
Diamond KR, Simons JP, Crawford AS, Judelson DR, Jones DW, Nguyen TT, Aiello FA and Schanzer A*
Department of Vascular and Endovascular Surgery, University of Massachusetts Memorial Medical Center, Worcester, MA, United States
- *Corresponding Author:
- Andres Schanzer
Division of Vascular and Endovascular Surgery, University of Massachusetts Memorial Medical Center,
Worcester, MA, United States
E-mail: Andres.Schanzer@umassmemorial.org
Received date: 03 January, 2022, Manuscript No. IPJVES-22-12423; Editor assigned date: 05 January, 2022, PreQC No. IPJVES-22-12423(PQ);
Reviewed date: 17 January, 2022, QC No IPJVES-22-12423; Revised date: 24 January, 2022, Manuscript No. IPJVES-22-12423(R);
Published date: 31 January, 2022, DOI: 10.366482634-7156.7.1.4601
Citation: Diamond KR, Simons JP, Crawford AS, Judelson DR, Jones DW, et al. (2022). Outcomes of Percutaneous-First Femoral Artery Access for Fenestrated and Branched Endovascular Aneurysm Repair. Int J Vasc Endovasc Therapy Vol.7 No.1:4601
Abstract
Introduction: Percutaneous femoral artery access is often employed for infrarenal Endovascular Aneurysm Repair (EVAR) given its safety, decreased length of stay and improved perioperative quality of life compared to open femoral artery exposure. However, evaluation of percutaneous femoral artery outcomes for Fenestrated and Branched EVAR (F/BEVAR) is limited. We sought to compare outcomes of a percutaneous-first femoral artery access strategy with an open-first femoral artery access strategy for F/BEVAR.
Methods: We reviewed a single-institution, prospectively maintained database of all F/BEVAR procedures performed as part of an FDA-approved PS-IDE trial (G130210) from 2013-2021. Patients were divided into two groups based on femoral artery access strategy: (1) Percutaneous-first–all patients treated with a percutaneous first approach (we adopted a percutaneous-first strategy for all F/BEVAR procedures in 08/2019) and (2) open-first-all patients with planned open femoral artery exposure. Covariates included patient demographics, medical comorbidities, and aneurysm-specific characteristics. Our primary endpoint was access site complication (thrombosis, hematoma requiring transfusion/intervention, pseudoaneurysm requiring intervention, groin infection or wound breakdown, or conversion to open femoral exposure). Secondary outcomes included technical success, Estimated Blood Loss (EBL), number of transfusions, 30-day perioperative outcomes (myocardial infarction, paraparesis, paralysis, stroke, acute kidney injury, dialysis, target artery occlusion, type 1 or 3 endoleak, and mortality), length of stay, and 30-day readmission. Cox proportional hazards modeling was used to assess the independent effect of percutaneous access on 1-year mortality.
Results: From 12/2013-10/2020, 259 consecutive F/BEVAR procedures were performed with 201 (78%) in the open-first group and 58 (22%) in the percutaneous-first group. Between groups, there were no differences in baseline demographics, BMI, history of open or endovascular aortic surgery, previous surgical groin exposure, aneurysm extent, urgency of repair, number of target arteries, device type, or number of stents placed (all P>.05). Access site complications did not differ between groups (5.2% vs. 2.5%, P=.57). Among the percutaneous-first group, 8(13.5%) required conversion to open femoral artery exposure. However, EBL (381 cc vs. 476 cc, P=.049), 30-day readmission (11% vs. 28%, P=.02), time from incision to closure (3.3 hours vs. 4.1 hours, P<.0001) and radiation dose (4499 mGy vs. 5550 mGy, P=.04) were all significantly lower for percutaneous-first procedures. Contrast volume (89 mL vs. 64 mL, P<.0001) was higher for percutaneous-first procedures. On multivariable analysis, femoral access strategy was not associated with one-year mortality.
Conclusion: Percutaneous femoral access for F/BEVAR is a safe alternative to open femoral exposure with comparable rates of access site complications and perioperative outcomes. Blood loss, operating room time, and readmissions are reduced with a percutaneous-first strategy, suggesting improved resource utilization compared to an open-first femoral access strategy.
Keywords
Thoracoabdominal aneurysm; EVAR FEVAR; Percutaneous EVAR pEVAR
Introduction
Endovascular Aortic Repair (EVAR) has revolutionized the field of aortic surgery. Historically, open surgical repair necessitated a prolonged inpatient hospitalization, admission to the critical care unit, and posed significant morbidity and mortality. The introduction of EVAR has resulted in favorable early morbidity and mortality for the repair of Abdominal Aortic Aneurysms (AAA) [1-3]. Moreover, the treatment of complex paravisceral and thoracoabdominal aortic aneurysms with Fenestrated and Branched EVAR (F/BEVAR) has seen a parallel, if not more pronounced, improvement in perioperative outcomes when compared with open surgical repair [4-7].
A percutaneous approach to infrarenal EVAR offers patients decreased time to ambulation, decreased pain, and fewer wound complications. Additionally, percutaneous EVAR has also been shown to reduce operative time, decrease length of stay, and offer a high rate of technical success [8-10]. Together, these facets have made percutaneous EVAR an attractive alternative to open surgical femoral artery exposure for the delivery of the endograft. It is no surprise that there has been increasing adoption of this technique nationwide.
Unlike infrarenal EVAR, the F/BEVAR experience in the United States has been limited to a relatively small number of sites, often in the context of FDA physician-sponsored investigation device exemption trials. These procedures often involve large-bore contralateral femoral artery access, steerable sheaths, and multiple flexible sheaths for the cannulation of target visceral arteries. Although percutaneous EVAR has been repeatedly demonstrated to be effective for the treatment of infrarenal AAAs, less is known about whether percutaneous F/BEVAR is safe for the treatment of complex paravisceral and Thoracoabdominal Aortic Aneurysms (TAAA). In this study, we compare a percutaneous-first femoral artery access strategy to open-first surgical femoral artery exposure for F/BEVAR.
Methods
Study design
This is a single-center, prospective observational cohort study. All data were collected prospectively as part of a physician-sponsored investigational device exemption trial (PS-IDE, FDA ID# G130210) of physician modified endografts and company manufactured custom and off-the-shelf devices. Institutional review board approval was obtained from the university of Massachusetts medical school. Each patient provided written informed consent.
Patient cohort
All patients undergoing fenestrated or branched endovascular aneurysm repair (>1 fenestration and/or branch) for the repair of an aortic arch aneurysm, thoracoabdominal aortic aneurysm, pararenal abdominal aortic aneurysm, or juxtarenal abdominal aortic aneurysm were included in the present study. All patients were previously deemed too-high-risk for open repair of their aneurysm, as determined by the surgeon and study team. Procedures were planned based on high-resolution Computed Tomography Angiography (CTA). Each study was reviewed on a three-dimensional workstation. Centerline-reconstruction was then utilized to obtain orthogonal measurements (TeraRecon, foster city, CA) to determine candidacy for stent-graft repair. Custom-made, commercially manufactured fenestrated and/or branched devices were utilized. For patients with anatomy suitable for a commercially approved fenestrated or branched device available to the study team (i.e. Zenith Fenestrated 9ZFEN)), or off-the-shelf trial device (i.e., Cook p-Branch), the corresponding commercially manufactured device was chosen and the patient was not included in the PS-IDE trial. For patients whose aneurysms were deemed too high risk to wait the required manufacturing time (i.e. symptomatic or ruptured aneurysms) and whose anatomies were not appropriate for an off-the-shelf device (i.e., Cook t-Branch), a Physician-Modified Endograft (PMEG) was used.
All patients undergoing F/BEVAR for complex aneurysms during the study period were eligible for inclusion. Prior to August 2019, all patients who underwent endovascular repair of complex aneurysms did so via open surgical exposure of the common femoral arteries. Beginning August 1st, 2019, we adopted a percutaneous-first approach to femoral artery access for all patients undergoing F/BEVAR. The surgeon would attempt percutaneous bilateral femoral artery access. If percutaneous access or femoral artery pre-closure could not be performed, the surgery would proceed via unilateral or bilateral femoral artery open surgical exposure. In the case of conversion to either unilateral or bilateral femoral artery open surgical exposure, the patient was considered a conversion to open.
Covariates examined
Patient demographics included patient age, gender, and body mass index (BMI, kg/m2). Medical comorbidities examined included coronary artery disease, cerebrovascular disease (stroke or transient ischemic attack), hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease (defined as baseline creatinine>1.8 mg/dL), history of cancer (any), and smoking history (current, former, or never). Aortic-specific covariates included history of open aortic surgery, previous EVAR, aneurysm extent (Extent I-IV, juxtarenal, pararenal), aneurysm diameter (maximal cross-sectional diameter measured by centerline reformatting), and procedural urgency (elective, urgent, or emergent).
Study Design
The study population was stratified into one of two groups; (1) Percutaneous-first (“Percutaneous”), defined as all patients who underwent attempted percutaneous femoral artery access for their repair, including those who underwent conversion from a percutaneous approach to an open surgical exposure (repairs taking place after August 1st, 2019), and (2) open-first (“Open”), defined as all patients undergoing a planned open femoral artery exposure (repairs taking place prior to August 1st, 2019).
Primary and secondary endpoints
Our primary endpoint was access site complication, defined as access site thrombosis, hematoma requiring transfusion and/or return to the operating room, pseudoaneurysm requiring intervention (percutaneous thrombin injection or operative repair), groin infection, groin wound breakdown, or conversion to open femoral exposure. Technical success, Estimated Blood Loss (EBL), number of transfusions, 30-day perioperative adverse events, length of stay and 30-day readmission were included as secondary outcomes. Technical success was defined as successful delivery of the endograft with cannulation of all intended target arteries and absence of type 1 or 3 endoleak on completion angiography. Estimated blood loss was recorded for each procedure in milliliters. Number of transfusions was reported as a combination of units of packed red blood cells transfused in the operating room and during the patient’s hospitalization. Perioperative adverse events included myocardial infarction (defined according to the American Heart Association’s universal definition of myocardial infarction), paraparesis (baseline change in lower extremity strength as determined by the surgical team), paralysis (complete loss of motor function in the lower extremities), cerebrovascular accident (transient ischemic attack or stroke), acute kidney injury (defined as increase in baseline serum creatinine >0.3 mg/dL or >30% increase above baseline), new-onset need for renal replacement therapy, target artery patency, type 1 or 3 endoleak on completion angiography, and perioperative mortality (30-days).
Intraoperative and postoperative management
Femoral artery access was obtained via open surgical exposure or percutaneous approach. For open surgical exposure, a transverse groin incision parallel to the inguinal ligament was utilized whenever possible to assist with wound healing. In the usual standard fashion, the common femoral, superficial femoral, and deep femoral arteries were controlled with vessel loops prior to introduction of an 18-gauge access needle, stiff wire, and the fenestrated endograft and contralateral sheath. Special circumstances, namely common femoral occlusive disease, necessitated endarterectomy and patch angioplasty at the discretion of the operating surgeon. However, whenever possible, the arteriotomy was repaired primarily with interrupted polypropylene sutures. The surgical wound was closed using multiple layers of vicryl suture to approximate the femoral sheath and subcutaneous tissues. The skin was closed with monocryl suture and dressed with surgical adhesive.
Percutaneous femoral artery access was achieved via the pre-close method. The operator obtained ultrasound-guided access to the common femoral arteries bilaterally. After wire selection of the aorta, two perclose proglide (Abbott, Abbott Park, IL) suture-mediated closure devices were deployed at the two o’clock and ten o’clock positions. The sutures were clamped with a hemostat and left in place until the conclusion of the procedure. Upon completion of the repair, a knot pusher was used to advance the suture. Once the wound was hemostatic, the guidewire was removed from the access site and the knot was tightened and trimmed per the device’s Instructions for Use.
Patients with long-segment aortic coverage extending >4 cm superior to the celiac artery, occluded hypogastric or vertebral arteries, or otherwise deemed at high-risk for Spinal Cord Ischemia (SCI) underwent attempted prophylactic lumbar drainage by a cardiovascular anesthesiologist and/or neuro-interventional radiologist. Additionally, selective placement of a lumbar drain was utilized after previous open or endovascular aortic reconstructions, depending on the extent of prior repair and disruption of intercostal collaterals to the spinal cord. These patients were drained, as per a set protocol, in a dedicated heart-and-vascular intensive care unit. Additional adjuncts were employed to prevent SCI. A hematocrit goal of greater than 30% was utilized in the perioperative period. In addition to spinal drainage, patients at high risk for SCI underwent Mean Arterial Blood Pressure (MAP) augmentation during the perioperative period with a MAP goal of greater than 90 mmHg utilized for each patient through postoperative day 1. If the patient developed SCI, this goal was increased to greater than 110 mmHg. If the patient did not develop SCI, the MAP goal was decreased to greater than 70 mmHg on postoperative day 2. Staging of different repair steps (i.e. first stage TEVAR, iliofemoral bypass conduit, carotid-subclavian bypass) was also utilized whenever possible to minimize the risk of SCI.
Follow-up
All patients were followed with serial imaging as per our institution’s standardized protocol. Patients underwent CTA at 1 month, 6 months, and annually thereafter. Patients with contraindications to serial CTA were imaged with non-contrast CT scans and serial Duplex Ultrasound (DUS) at the same time intervals.
Statistical Analyses
Descriptive statistics were used to compare demographics, types of repair, operative characteristics, and outcomes between groups. Chi-square and t-tests were used as appropriate for univariate analyses. Cox proportional hazards modeling was used to identify whether access strategy (percutaneous-first vs. open-first) was independently predictive of one-year mortality after adjusting for a pre-determined set of potential confounders (age, history of coronary artery disease, aneurysm diameter, procedural urgency, baseline renal dysfunction). Importantly, all analyses were conducted based on access strategy, even if a percutaneous patient was converted to open femoral artery exposure. An alpha level of 0.05 was used to determine statistical significance. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
Results
Cohort description
Between January 1st, 2013 and December 30th, 2020, 259 consecutive F/BEVAR procedures were performed (Table 1). Of these, 201 (78%) were performed with planned open femoral artery access (prior to August 1, 2019), while 58 (22%) were performed with a percutaneous-first approach (after August 1, 2019). The cohort consisted of a majority of men (n=182, 70%) with an average age of 74, and a mean Body Mass Index (BMI) of 27. Of the medical comorbidities examined, medical history of coronary artery disease (31% vs. 48%, P=.02) was more common in the percutaneous group, while current tobacco use (19% vs. 29%, P=.03) were more common in the open group. Between groups, there were no differences in history of previous aortic surgery, previous EVAR, previous femoral artery surgery, aneurysm extent, or procedural urgency between groups. Mean maximum aneurysm sac diameter was larger for the open group, compared to the percutaneous group (66 mm vs. 62 mm, P=.02).
| Total (N=259) | Percutaneous | Open | P | |
|---|---|---|---|---|
| (N=58) | (N=201) | |||
| Age, mean (SD) | 74 (8.5) | 74 (8.3) | 74 (8.6) | 0.94 |
| Male gender | 182 (70) | 46 (79) | 136 (68) | 0.09 |
| Comorbidities | ||||
| Coronary artery disease | 115 (44) | 18 (31) | 97 (48) | 0.02 |
| Cerebrovascular disease | 32 (12) | 8 (14) | 24 (12) | 0.67 |
| Hypertension | 250 (97) | 57 (98) | 193 (96) | 0.69 |
| Hyperlipidemia | 240 (93) | 57 (98) | 183 (91) | 0.08 |
| Diabetes mellitus | 41 (16) | 11 (19) | 30 (15) | 0.46 |
| Chronic obstructive pulmonary disease | 98 (38) | 21 (36) | 77 (38) | 0.77 |
| Renal failure requiring dialysis | 5 (1.9) | 0 (0) | 5 (2.5) | 0.59 |
| Previous diagnosis of cancer | 74 (29) | 13 (22) | 61 (30) | 0.24 |
| Current smoking | 69 (27) | 11 (19) | 58 (29) | 0.03 |
| Body mass index, mean (SD) | 27 (5.2) | 27 (4.8) | 27 (5.3) | 0.74 |
| Prior aortic surgery | 112 (43) | 31 (53) | 81 (40) | 0.07 |
| Prior EVAR | 50 (19) | 15 (26) | 35 (17) | 0.15 |
| Aneurysm extent | ||||
| Juxtarenal aortic aneurysm | 88 (34) | 25 (43) | 63 (31) | 0.1 |
| Pararenal aortic aneurysm | 19 (7.3) | 1 (1.7) | 18 (9.0) | 0.08 |
| Extent type 1 TAAA | 2 (0.8) | 0 (0) | 2 (1.0) | 0.99 |
| Extent type 2 TAAA | 33 (13) | 5 (8.6) | 28 (14) | 0.29 |
| Extent type 3 TAAA | 53 (20) | 11 (19) | 42 (21) | 0.75 |
| Extent type 4 TAAA | 53 (20) | 10 (17) | 43 (21) | 0.49 |
| Extent type 5 TAAA | 6 (2.3) | 3 (5.2) | 3 (1.5) | 0.13 |
| Aortic arch aneurysm | 1 (0.4) | 0 (0) | 1 (0.5) | 0.99 |
| Max aneurysm diameter (mm) | 65 (12) | 62 (8.5) | 66 (12) | 0.02 |
| Urgency | 0.47 | |||
| Elective intact aneurysm | 236 (96) | 57 (98) | 179 (95) | |
| Urgent symptomatic aneurysm | 11 (4.5) | 1 (1.7) | 10 (5.3) | |
| Emergent | 0 (0) | 0 (0) | 0 (0) |
Table 1: Cohort demographics
Procedural outcomes
Between groups, there were no differences in the type of fenestrated or branched devices utilized (P=.06), mean number of target arteries per patient (3.7 vs. 3.6, P=.27), or mean number of stent grafts placed per patient (P=.66) (Table 2). There was a greater average number of fenestrations utilized during percutaneous repairs (2.8 per patient vs. 2.2 per patient, P=.005), but no differences in the mean number of branches (0.1 vs. 0.2, P=.10) or scallops (0.7 vs. 0.9, P=.28).
| Total | Percutaneous | Open | P | |
|---|---|---|---|---|
| (N=259) | (N=58) | (N=201) | ||
| Device type | 0.6 | |||
| Custom made device | 237 (92) | 58 (100) | 179 (90) | |
| Physician modified endograft | 16 (6) | 0 (0) | 16 (7) | |
| T-Branch | 3 (1) | 0 (0) | 3 (2) | |
| P-Branch | 1 (1) | 0 (0) | 1 (1) | |
| Target arteries per patient | 3.6 (0.7) | 3.7 (0.6) | 3.6 (0.7) | 0.27 |
| Bridging stent grafts per patient | 0.66 | |||
| One | 5 (2) | 1 (2) | 4 (2) | |
| Two | 12 (5) | 1 (2) | 11 (6) | |
| Three | 63 (25) | 13 (22) | 50 (25) | |
| Four or more | 177 (69) | 43 (74) | 134 (67) | |
| Branches | 0.2 (0.4) | 0.1 (0.3) | 0.2 (0.4) | 0.1 |
| Fenestrations | 2.3 (1.5) | 2.8 (1.5) | 2.2 (1.5) | 0.005 |
| Scallops | 0.8 (1.4) | 0.7 (1.2) | 0.9 (1.4) | 0.28 |
Table 2: Repair characteristics for all patients undergoing F/BEVAR.
There were no differences in the number of transfusions (mean 1.3 vs. 1.0, P=.38), overall technical success (97% vs. 96%, P=.99), ICU length of stay (mean 1.6 days vs. 1.6 days, P=.98), or overall hospital length of stay (mean 4.0 days vs. 4.8 days, P=.21) when stratified by access strategy (Table 3). For the percutaneous group, there was a decreased amount of radiation (mean 4499 mGy vs. 5550 mGy, P=.04), estimated blood loss (mean 381 cc vs. 476 cc, P=.05), operative time (incision-to-closure, mean 3.3 hours vs. 4.1 hours, P<.0001), and rehospitalization within 30-days (8.6% vs. 24%, P=.02). Contrast use was increased in the percutaneous group (mean 89 cc vs. 64 cc, P<.0001). Successful femoral access with successful delivery of the fenestrated component was not statistically different between groups (96% vs. 100%, P=.38), however, there were eight patients (13.8%) in the percutaneous group who experienced failed percutaneous femoral artery access requiring conversion to open surgical exposure.
| Total | Percutaneous | Open | P | |
|---|---|---|---|---|
| (N=259) | (N=58) | (N=201) | ||
| Exam dose (mGy) | 5331 (3278) | 4499 (2716) | 5550 (3383) | 0.04 |
| Volume of contrast used (mL) | 69 (33) | 89 (29) | 64 (32) | <.0001 |
| Estimated blood loss (cc) | 457 (357) | 381 (278) | 476 (372) | 0.05 |
| Number of transfusions | 1.1 (1.7) | 1.3 (1.7) | 1.0 (1.7) | 0.38 |
| Operative time (hours) | ||||
| Incision to surgery end | 3.9 (1.2) | 3.3 (1.1) | 4.1 (1.2) | <.0001 |
| Technical success | ||||
| Yes | 248 (96) | 56 (97) | 192 (96) | 0.99 |
| No | 11 (4.3) | |||
| Successful femoral access (n, %) | 251 (97) | 50 (86) | 201 (100) | 0.38 |
| ICU length of stay (mean, SD) | 1.6 (4.5) | 1.6 (2.3) | 1.6 (5.0) | 0.98 |
| Hospital length of stay (mean, SD) | 4.6 (5.2) | 4.0 (3.6) | 4.8 (5.6) | 0.21 |
| Re-hospitalization within 30 days (N, %) | 54 (21) | 5 (8.6) | 49 (24) | 0.016 |
Table 3: Technical outcomes following F/BEVAR.
Perioperative outcomes
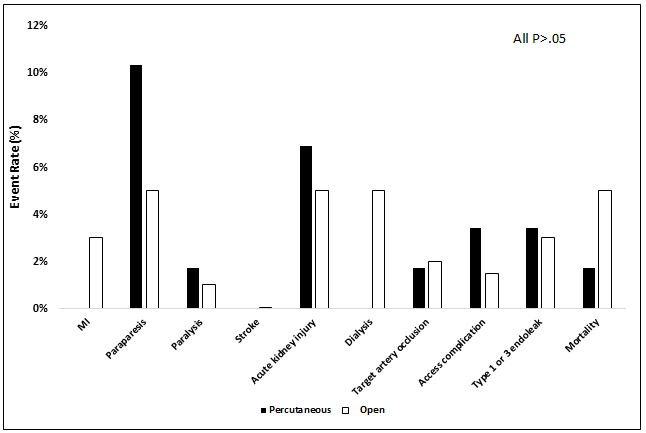
On review of 30-day perioperative outcomes, there were no differences in rates of myocardial infarction (0% vs. 3.0%, P=.34), paraparesis (10.3% vs. 5.0%, P=.21), paralysis (1.7% vs. 1.0%, P=.53), stroke (0% vs. 0.1%, P=.99), acute kidney injury (6.9% vs. 5.0%, P=.53), dialysis (0% vs. 5.0%, P=.12), target artery occlusion (1.7% vs. 2.0%, P=.99), access site complications (3.5% vs. 1.5%, P=.31), type 1 or 3 endoleak (3.5% vs. 3.0%, P=.99), or mortality (1.7% vs. 5.0%, P=.46) (Figure 1).
Access site complications
Although there were no differences between groups in rates of access site complications following F/BEVAR (P=.31), the types of complications were different. In the open group, there were four patients with groin complications after femoral artery exposure (4/201, 2%) (Table 4). Two patients suffered groin infections diagnosed 81 and 111 days after their index operation. Each patient underwent incision and drainage and required inpatient admission for three days for intravenous antibiotics. Both patients were discharged home with negative pressure wound therapy and close interval follow-up in the vascular surgery clinic. One patient in the open group had dehiscence of a groin wound 16 days after operation. This was treated with local wound care and close observation and did not require hospital admission or operative intervention. The last patient required a return to trip to the operating room for acute limb ischemia due to a thrombosed common femoral artery diagnosed on post-operative day one. Unfortunately, this patient had a relatively prolonged course complicated by a groin infection requiring multiple trips to the operating room for wound debridement and intravenous antibiotics. Each patient was alive thirty-days after F/BEVAR and no patient was readmitted within thirty days.
| ID | Complication | Intervention(s) | Time to complication | Readmitted within 30-days | Alive at 30 days |
|---|---|---|---|---|---|
| 1 | Groin infection | Incision and drainage, admission x 3 days for IV antibiotics | 81 days | No | Yes |
| 2 | Groin infection | Incision and drainage, admission x3 days for IV antibiotics | 111 days | No | Yes |
| 3 | Groin wound dehiscence | Local wound care | 16 days | No | Yes |
| 4 | Acute limb ischemia, subsequent groin infection | Femoral thrombectomy, patch angioplasty, Wound debridement | 1 day | No | Yes |
Table 4: Groin complications for open femoral artery access patients.
Eight patients in the percutaneous-first group underwent conversion from percutaneous femoral access to open surgical exposure (8/58, 14%) (Table 5). Patient one underwent a right groin cutdown at the time of the fenestrated repair due to the presence of a previous lower extremity bypass. At the time of their operation, there was insufficient length of healthy common femoral artery to safely proceed with a percutaneous access strategy. Patients two, six, and eight all had significant scarring due to previous surgeries and/or percutaneous interventions, such that the perclose device could not be introduced. Each patient required bilateral femoral artery cutdowns. Patient four was morbidly obese (BMI=51 kg/cm2) and the perclose device was not long enough, precluding safe percutaneous femoral access and closure. Finally, patient five was noted to have perclose device failure during vessel closure. This required replacement of the femoral sheath and subsequent cutdown for vessel repair.
| ID | Femoral cutdown | Reason | Groin complication | Intervention | Readmission 30-days | Reason for Readmission | Alive at 30-days |
|---|---|---|---|---|---|---|---|
| 1 | Unilateral | Previous lower extremity bypass | No | No | Yes | ||
| 2 | Unilateral | Scar tissue, unable to track closure device | No | No | Yes | ||
| 3 | Bilateral | Atherosclerosis, thrombosis of common femoral artery | Yes, Acute limb ischemia | Femoral thrombectomy | No | Yes | |
| 4 | Unilateral | Morbid obesity | No | No | Yes | ||
| 5 | Unilateral | Closure device failure | No | No | Yes | ||
| 6 | Bilateral | Scar tissue, unable to track closure device | No | Yes | Acute kidney injury | Yes | |
| 7 | Unilateral | Scar tissue, unable to track closure device | No | No | Yes | ||
| 8 | Bilateral | Scar tissue, unable to track closure device | No | No | Yes |
Table 5: Percutaneous access strategy patients requiring conversion to open femoral artery exposure.
Two additional patients within the percutaneous group suffered groin-access related complications (2/58, 3%). Patient three was noted to have a change in pedal pulse exam immediately following percutaneous vessel closure. This patient required unilateral femoral cutdown with thrombectomy, endarterectomy, and patch angioplasty. This resulted in a return of palpable pedal pulses and no long-term sequelae after open conversion. Patient eight was noted to have a rapidly expanding groin hematoma on post-operative day one requiring application of manual pressure and a transfusion of one-unit of packed red blood cells to support their augmented MAP and hematocrit goals.
Importantly, all patients who crossed-over from a percutaneous-first access strategy to open surgical exposure were alive at thirty-days. Additionally, there were no groin infections or groin wound dehiscence within this subset. Only one patient required readmission. Patient six was readmitted to an outside institution from home three days after discharge with acute kidney injury due to dehydration. The patient was discharged 48 hours later when their creatinine returned to baseline.
One-year mortality
One-year mortality for the entire cohort was 14.5% and did not differ between the percutaneous and open groups (9.3% vs. 16%, P=.22). On cox proportional hazards modeling of one-year mortality, femoral artery access strategy was not found to be an independent determinant of one-year mortality (HR=0.64, 95% CI 0.24-1.73, P=.38) (Table 6). Similarly, age (HR=1.04, 95% CI 0.99-1.08, P=.08), history of coronary artery disease (HR=0.68, 95% CI 0.34-1.35, P=.27), aneurysm diameter (HR=0.99, 95% CI 0.97-1.03, P=.94), and urgent procedural status (HR=1.67, 95% CI 0.34-8.16, P=.53) were not associated with one-year mortality. However, baseline creatinine >1.8 mg/dL was independently predictive of one-year mortality (HR=2.84, 95% CI 1.14-7.09, P=.03).
| Hazard ratio | 95% CI | P | |
|---|---|---|---|
| Percutaneous-first femoral access | 0.64 | 0.24-1.73 | 0.38 |
| Age (per additional year) | 1.04 | 0.99-1.08 | 0.08 |
| History of coronary artery disease | 0.68 | 0.34-1.35 | 0.27 |
| Aneurysm diameter (per additional cm) | 0.99 | 0.97-1.03 | 0.94 |
| Urgent procedural status (versus elective) | 1.67 | 0.34-8.16 | 0.53 |
| Baseline creatinine>1.8mg/dL | 2.84 | 1.14-7.09 | 0.03 |
Table 6: Cox proportional hazards modeling of one-year mortality following F/BEVAR.
Discussion
Surgical femoral artery exposure had previously been the standard of care for EVAR. Over the last ten years, there has been a transition away from surgical femoral cutdown towards percutaneous femoral access for EVAR [11]. For infrarenal repairs, there is a large body of literature that supports a percutaneous-first approach to femoral access with excellent technical outcomes and enhanced patient-reported quality of life metrics, especially in the perioperative period [8,9] In their 2013 study, Nelson et al. reported the first multicenter randomized controlled trial designed to assess the safety and effectiveness of percutaneous EVAR. Their non-inferiority study demonstrated that procedural success was high and offered patients improved outcomes related to blood loss, pain, and quality of life. Later, Buck et al. performed a review of 4112 percutaneous and open EVAR procedures performed over a two-year period in the ACS NSQIP database [12]. The authors of this study found that not only did percutaneous EVAR result in shorter operative times, but also offered a shorter length of stay and fewer wound complications. These results have led to the widespread adoption of percutaneous EVAR worldwide.
While principles of EVAR may be applied to more complex F/BEVAR, there are technical aspects to these procedures that may present challenges for a percutaneous approach to femoral artery access. While low-profile fabrics, nitinol stent architecture, and other improvements in device engineering have resulted in the introduction of lower-profile devices, custom-made company manufactured devices and physician modified endografts are inherently large-bore devices and are often 18 French or greater in diameter. The addition of pre-loaded wires/catheters to their delivery systems facilitates efficient target vessel cannulation but leads to larger diameter devices for these repairs. Furthermore, additional techniques required of these repairs include multiple punctures in the contralateral sheath for the cannulation of the desired target arteries and delivery of multiple stent grafts. As a result, at our institution we began our initial F/BEVAR experience with routine surgical femoral artery exposure to limit the impact of access site complications on the procedural success. With greater experience and increased comfort level with the procedure as a whole, we later transitioned to a percutaneous-first approach for all F/BEVAR, similar to our experience with infrarenal EVAR.
To our knowledge, this is the largest study comparing outcomes of a percutaneous-first femoral artery access strategy to open surgical exposure in F/BEVAR. In our study, we found that there were no differences in overall rates of access site complications, technical success, or major adverse events at 30-days, when stratified by access strategy. Additionally, percutaneous femoral access was associated with decreased radiation dose, estimated blood loss, operative time, and decreased hospital readmission within 30 days. These findings were observed in a patient cohort of similar demographics, aneurysm morphologies, and overall technical complexity as evidenced by a similar number of target arteries incorporated in repairs.
In an effort to limit healthcare costs and evaluate quality of care, attention has been directed towards the development of clinical programs to reduce unplanned readmission within thirty-days. Following the introduction of the affordable care act, many payors now penalize institutions for unplanned readmissions, which are often costly and more frequent following vascular surgery [13]. As a result, significant attention has been directed towards reducing these events. In our study, we found the overall rate of readmission following F/BEVAR was 21%. This is significantly higher than that of the literature focused on infrarenal EVAR. In their 2019 study, Dua et al. reported 30-day readmissions following infrarenal EVAR was 11.6% within the nationwide readmissions database. EVAR readmissions were independently associated with younger patients, female sex, insurer, urgent procedures, and high-risk medical comorbidities (CHF, renal failure, complicated diabetes, and peripheral vascular disease). When comparing patient comorbidity-related readmissions to F/BEVAR, we found that there was nearly a threefold reduction in 30-day readmission with a percutaneous-first femoral artery access strategy for F/BEVAR (8.6% vs. 24%, P=.016), even though the cohort examined in our study represents a high-risk population. Given that patient comorbidity-related readmissions in our F/BEVAR cohort were similar to EVAR, it is likely that our observed reduced readmission rate in the percutaneous-first group is attributed to femoral artery access. This may be a function of decreased operative time, pulmonary complications, and surgical site infection risk. Taken in aggregate, these data suggest that at high volume centers with adequate procedural experience and institutional support, rates of readmission following F/BEVAR may be similar to that of infrarenal EVAR when a percutaneous-first approach to femoral artery access is employed [14].
Conversion from percutaneous to open femoral artery access occurred in eight patients. We reviewed each of these cases individually and reported the reasons for each conversion. Fortunately, there were few cases of occult closure device failure (n=2, 4%), similar to the ProGlide subset in the original pEVAR study [8]. Additionally, body habitus was a contributing factor for one patient in our series. The most common cause of conversion to open femoral exposure was significant groin scar tissue from previous femoral artery interventions, occurring in half of the crossover patients (n=4). In these patients, scar tissue was often so robust that delivery of a short 6 French sheath or the ProGlide device was not able to be performed. In these patients, the operating surgeon felt it to be safer to expose the femoral artery prior to continuing the procedure. Unfortunately, further review of each patient’s preoperative history, physical, and imaging did not lead to an identifiable preoperative variable predictive of scar tissue that precludes percutaneous femoral access. This remains an ongoing point of interest and an important clinic question that warrants further investigation.
Our study has limitations. First, while this database is prospectively maintained and reports consecutive patients undergoing F/BEVAR in a PS-IDE clinical trial, some of the granular details for this specific study were collected retrospectively. Additionally, while the present study reports on a cohort of 259 patients, there is still potential for type two error and inadequate power to demonstrate difference in outcomes where differences exist. This may be especially important for overall hospital length of stay, which was nearly one-day shorter for the percutaneous group. Finally, an important clinical question that is unaddressed by the present study is identifying preoperative variables predictive of failed percutaneous femoral artery access prior to F/BEVAR.
Conclusion
Percutaneous femoral artery access offers excellent patient-reported outcomes following infrarenal EVAR and has become the standard of care in many centers. Despite increasing procedural complexity and requirement of large-bore femoral access bilaterally, percutaneous femoral artery access for F/BEVAR appears to offer similar rates of technical success, access site complications, perioperative major adverse events, and one-year mortality as open femoral artery exposure. This, in combination with the benefits of reduced procedural time, blood loss, and lower rates of hospital readmission make percutaneous F/BEVAR an attractive alternative to open femoral artery exposure.
References
- Roger MG, Louise CB, Janet TP, Simon GT, David E, et al. (2010) Endovascular versus open repair of abdominal aortic aneurysm. N Eng J Med 362: 1863-71.
[Crossref], [Google Scholar], [Indexed]
- EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): Randomized controlled trial. Lancet 365: 2179-86.
[Crossref], [Google Scholar], [Indexed]
- Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, et al. (2008) Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Eng J Med 358: 464-74.
[Crossref], [Google Scholar], [Indexed]
- Diamond KR, Simons JP, Crawford AS, Arous EJ, Judelson DR, et al. (2021) The effect of thoracoabdominal aortic aneurysm extent on outcomes in patients undergoing fenestrated/branched endovascular aneurysm repair. J Vasc Surg 74: 833-842.
[Crossref], [Google Scholar], [Indexed]
- Cosselli JS, LeMaire SA, Preventza O, Delacruz KI, Cooley DA, et al. (2016) Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 151: 1323-1337.
[Crossref], [Google Scholar], [Indexed]
- Rigberg DA, Mcgory ML, Zingmond DS, Maggard MA, Agustin M, et al. (2006) Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: A statewide experience. J Vasc Surg 43: 217-222.
[Crossref], [Google Scholar], [Indexed]
- Schanzer A, Simons JP, Flahive J, Durgin J, Aiello FA, et al. (2017) Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg 66: 687-694.
[Crossref], [Google Scholar], [Indexed]
- Nelson PR, Kracjer Z, Kansal N, Rao V, Bianchi C, et al. (2014) A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg 59: 1181-93.
[Crossref], [Google Scholar], [Indexed]
- Torsello GB, Kasprzak B, Klenk E, Tassarek J, Osada N, et al. (2003) Endovascular suture versus cutdown for endovascular aneurysm repair: A prospective randomized pilot study. J Vasc Surg 38: 78-82.
[Crossref], [Google Scholar], [Indexed]
- Jeanbaptiste E, Hassenkhodja R, Haudebourg P, Bouillane PJ, Declemy S, et al. (2008) Percutaneous closure devices for endovascular repair of infrarenal abdominal aortic aneurysms: A prospective, non-randomized comparative study. Eur J Vasc Endovasc Surg 35: 422-428.
[Crossref], [Google Scholar], [Indexed]
- Siracuse JJ, Farber A, Kalish JA, Jones DW, Rybin D, et al. (2018) Comparison of access type on perioperative outcomes after endovascular aortic aneurysm repair. J Vasc Surg 55: 1554-61.
[Crossref], [Google Scholar], [Indexed]
- Buck DB, Karthaus EG, Soden PA, Ultee KH, van Herwaarden JA, et al. (2015) Percutaneous versus femoral cutdown access for endovascular aneurysm repair. J Vasc Surg 62: 16-21.
[Crossref], [Google Scholar], [Indexed]
- Jencks SF, Williams MV, Coleman EA (2009) Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 360: 1418-1428.
[Crossref], [Google Scholar], [Indexed]
- Dua A, Rothenberg KA, Wohlaer M, Rossi PJ, Lewis BD, et al. (2019) Unplanned 30-day readmissions after endovascular aneurysm repair: An analysis using the nationwide readmissions database. J Vasc Surg 70: 1603-1611.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences