The Foot Angiosomes as Integrated Level of Lower Limb Arterial Perfusion: Amendments for Chronic Limb Threatening Ischemia Presentations
Alexandrescu VA, Pottier M, Balthazar S and Azdad K
DOI10.21767/2573-4482.19.04.7
1Department of Vascular and Thoracic Surgery, Princess Paola Hospital, Marche-en- Famenne, Belgium
2Department of Anesthesiology, Princess Paola Hospital, Marche-en-Famenne, Belgium
3Department of Radiology, Princess Paola Hospital, Marche-en-Famenne, Belgium
- Corresponding Author:
- Vlad Adrian
Alexandrescu
Department of Vascular and Thoracic Surgery
Princess Paola Hospital, Marche-en-Famenne, Belgium
E-mail: v.alex@skynet.be
Received Date: January 30, 2019; Accepted Date: February 21, 2019; Published Date: February 28, 2019
Citation: Alexandrescu VA, Pottier M, Balthazar S , Azdad K. The Foot Angiosomes as Integrated Level of Lower Limb Arterial Perfusion: Amendments for Chronic Limb Threatening Ischemia Presentations. J Vasc Endovasc Therapy. Vol.4 No.1:6.
Copyright: © 2019 Alexandrescu VA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: The angiosome concept was initially pioneered by Taylor and Palmer in the plastic reconstructive surgery field. The authors described a reproducible model of arterial and venous distribution in humans that follows specific three-dimensional (3D) networks of tissue. The angiosome model yet represents a specific level among other staged and graduated levels of harmonious arterial irrigation in the lower extremity. Specific CLTI pathologies enhance characteristic arterial branches affectation, including the angiosomal source arteries. Evaluating main atherosclerotic lesions at peculiar Levels of arterial division may afford useful clinical information.
Method: The present study proposes a description of six levels of degressive arterial division and collateral distribution in the inferior limb, including the angiosomal stage. Following succeeding perioperative 2D angiographic observations over an eight-year period, these levels (I to VI) were analyzed (including the angiosomal Level III) and summarized in attached tables. The medical files of 323 limb-threatening ischemic foot wounds (Rutherford 4-6) in 295 patients (71% men) were retrospectively reviewed. All arterial patterns were defined by preoperative Angio-CT, or Angio-MRI, associating in each case perioperative angiography. For each anatomical Level the worse atherosclerotic lesions (TASC II) were located, then compared with adjacent levels in same individual CLTI patterns. For each ramification stage the heaviest occlusive angiographic lesions (TASC “C-D”) were selected and defined as “dominant”, in comparison with parallel “associate” atherosclerotic locations. A comparison between 196 diabetic, against 127 non-diabetic CLTI patterns of atherosclerotic occlusive disease was performed.
Results: Notable differences in the distribution of the dominant occlusive lesions were observed among the two Groups. Specific “Level I” iliac and common femoral lesions (10%), adding “Level II” superficial femoral (17%) also “Level II” femoro-popliteal (32%) dominant occlusive lesions in non-diabetics prevailed against correspondent iliac (3%), superficial femoral (7%), and femoro-popliteal (21%) occlusive lesions in diabetic CLTI subjects.
Conversely, “Level II” distal popliteal and tibial occlusions (33%), “Level III” pedal and angiosomal branches (34%) adding “Level III” dominant foot arches occlusions (5%) in diabetic patients, overruled correspondent popliteo-tibial (30%), pedal-angiosomal (5%), and isolated foot arches (0%) lesions in non-diabetics.
Conclusion: Inferior limb vasculature affords a harmonious 3D distribution of “tissueoriented” arterial axes including the angiosomal source arteries. These branches are coupled to a vast collateral compensatory system (choke-vessels, cutaneous perforators, and arterial-arterial interconnections) in a degressive array. Six Levels of arterial ramification (I-VI) can be described on angiographic analysis. To specific Levels I-II bear atherosclerotic occlusive disease location, characteristic Levels III-IV topographic occlusive disease is opposed in diabetic CLTI patients.
Keywords
Angiosome; Critical limb ischemia; Collateral circulation; Ulcer healing; Limb salvage; Diabetic ulcer
Abbreviations
ABI: Ankle-Brachial Index; AC: Angiosome Concept; AP: Ankle Pressure; ATA: Anterior Tibial Artery; CLI: Critical Limb Ischemia; CLTI: Chronic Limb Threatening Ischemia; CV: Choke-Vessels; CP: Cutaneous Perforators; DP: Dorsalis Pedis; DSA: Digital Subtraction Angiography; LP: Lateral Plantar; LC: Lateral Calcaneal; MC: Medial Calcaneal; MP: Medial Plantar; PT: Perforasome Theory; PTA: Posterior Tibial Artery; SPP: Skin Pressure Perfusion; TP: Toe Pressure; WTR: Wound- Targeted Revascularization
Introduction
The angiosome concept (AC) was first introduced in 1987 by Taylor and Palmer in the plastic reconstructive surgery field [1]. The authors analyzed the blood supply to the skin and deep tissues by precise dissection and radiographic assessment of human vessels in ex-vivo limb models [1]. The “angiosome entities” are essentially anatomical structures, named from the Greek “angeion” (meaning vessel), and “somite”, or “soma” (meaning section of the body) [1]. The initial study of Taylor correlates with previous anatomical data about topographical distribution of human vasculature. Following impressive number of dissections displayed over many years, the authors described a reproducible pattern of arterial and venous distribution in humans that follows specific three-dimensional (3D) networks of tissue layers [1]. Taylor et al. further extended the angiosome concept (AC) to assess other mammals and vertebrates and obtained results with remarkable similarities [2] The authors found notable correlations between the topographic vasculature in humans and vertebrates that reinforced initial observations of this novel anatomical concept [1,2].
The remarkable anatomical work of Taylor et al., further developed by Attinger et al., [3] is based on previous topographical descriptions on human regional vasculature [1-4]. More specifically, skin vascularization has incited early anatomical descriptions such as the notable topographical observations published by Manchot in 1889, and further developed by Salmon in 1936 on the same subject [1-4]. These authors initiated skin vasculature analysis by cadaver dissections adding X-ray contrast injection [4,5]. Following recent progresses in technology, the AC continues to incite further anatomical research on human tissue vasculature. In addition to the original forty-four angiosomes [1,4] more specific “source arteries” and related “cluster collaterals” have been identified in specific sectors of the human body [5-7].
Methods
Anatomical considerations
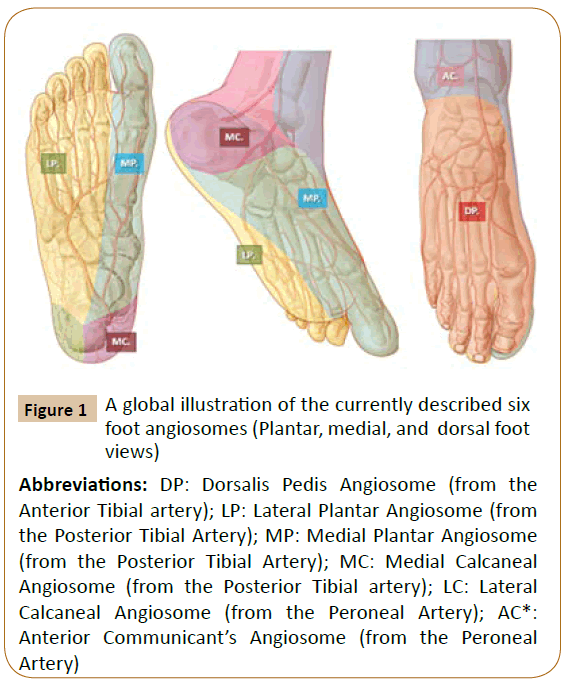
Specific source arteries of angiosomes: In their initial studies, Taylor and Palmer described the body blood supply in humans as a “continuous 3D network” of vessels in the skin and adjacent tissues [1]. These 3D blocks of skin and deep tissue are nourished by specific source arteries (Figures 1-3) and these findings were proved to be reproducible in most individuals [1,4,5]. These nourishing arteries vary in length, density, and caliber in different regions of the human body. Concerning the inferior limb, Taylor and colleagues observed that the skin, underlying bones, and most adjacent muscles received arterial branches from two or three neighbouring angiosomes [1,4]. The authors revealed the importance of various types of angiosomal interconnections in neighbouring compartments of the leg, as to compensate disruptions from ischemic disease or trauma in specific “source arteries” [1,4]. Interestingly, following this remarkable anatomical work, muscles in the anterior compartment of the leg and in the dorsal foot were supplied by only one angiosome [5].
Figure 1: A global illustration of the currently described six
foot angiosomes (Plantar, medial, and dorsal foot
views)
Abbreviations: DP: Dorsalis Pedis Angiosome (from the
Anterior Tibial artery); LP: Lateral Plantar Angiosome (from
the Posterior Tibial Artery); MP: Medial Plantar Angiosome
(from the Posterior Tibial Artery); MC: Medial Calcaneal
Angiosome (from the Posterior Tibial artery); LC: Lateral
Calcaneal Angiosome (from the Peroneal Artery); AC*:
Anterior Communicant’s Angiosome (from the Peroneal
Artery)
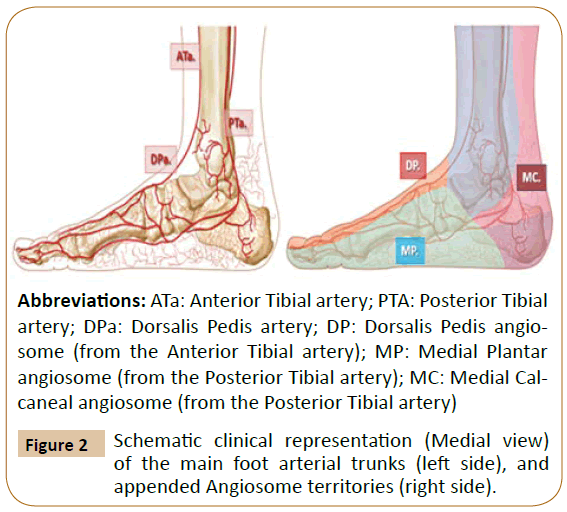
Abbreviations: ATa: Anterior Tibial artery; PTA: Posterior Tibial
artery; DPa: Dorsalis Pedis artery; DP: Dorsalis Pedis angiosome
(from the Anterior Tibial artery); MP: Medial Plantar
angiosome (from the Posterior Tibial artery); MC: Medial Calcaneal
angiosome (from the Posterior Tibial artery)
Figure 2: Schematic clinical representation (Medial view)
of the main foot arterial trunks (left side), and
appended Angiosome territories (right side).
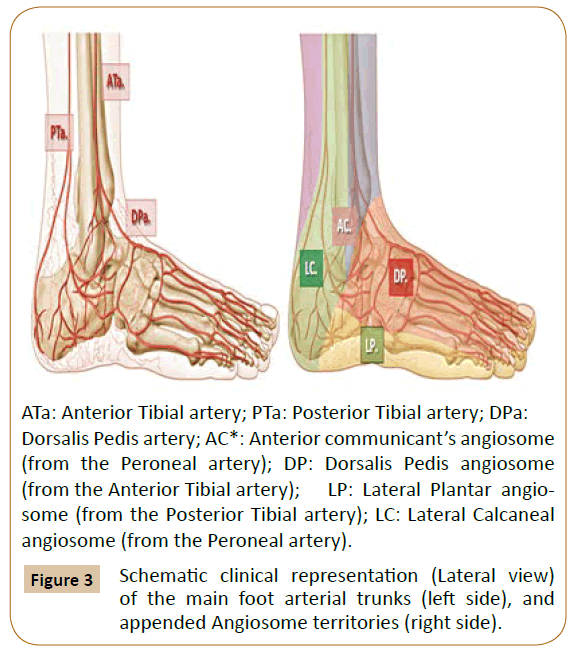
ATa: Anterior Tibial artery; PTa: Posterior Tibial artery; DPa:
Dorsalis Pedis artery; AC*: Anterior communicant’s angiosome
(from the Peroneal artery); DP: Dorsalis Pedis angiosome
(from the Anterior Tibial artery); LP: Lateral Plantar angiosome
(from the Posterior Tibial artery); LC: Lateral Calcaneal
angiosome (from the Peroneal artery).
Figure 3: Schematic clinical representation (Lateral view)
of the main foot arterial trunks (left side), and
appended Angiosome territories (right side).
These, and previous anatomical findings may contribute to explain the multifaceted clinical presentations of CLI due to various degrees of tibial atherosclerotic disease contained in rigid fascial anatomical compartments [3,8].
Associated angiosomal interconnections: “Choke-vessels”, “arterial-arterial” communicants, and “cutaneous perforators”. Neighboring angiosomes are surrounded by numerous small arterial branches named choke vessels (CV) [1,4]. These CVs connect adjacent angiosomes to one another and demarcate the border of each angiosome from peripheral tissues [1-4]. The global CV system creates an efficient compensatory network to enable the one source artery to supply several neighboring angiosomes [1,5]. In cases when a source artery is interrupted, severely narrowed, or occluded, this “rescue system” redirects the blood flow through available CVs towards the linked 3D tissue blocks. This notable flow support afforded by the CVs may also to be time-dependent [3,5,8] and closely associated with arteriogenic and angiogenic processes [4,8]. Since CVs encompass a myriad of small vessels connections, parallel larger diameter arterial-arterial connections and collaterals equally contribute to reorienting the compensating flow between regional foot angiosomes [1-5].
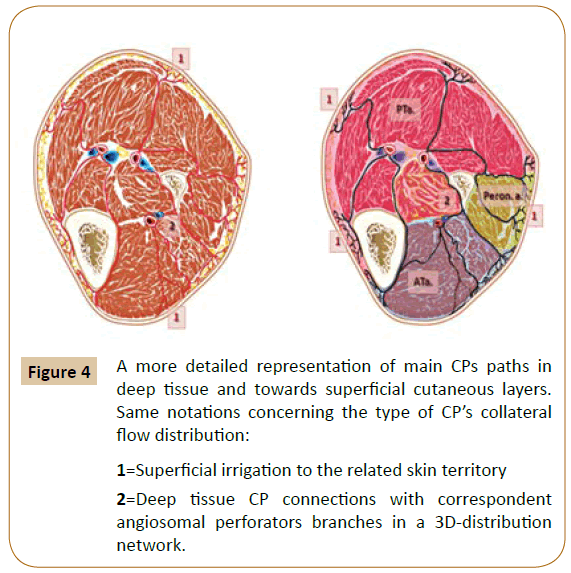
According to initial descriptions by Taylor et al. major skin territories receive equally important topographic perfusion via the “source arteries” and through at least three distinct levels of appended collaterals [1]. These ranks are represented by a huge amount of collateral “choke-vessels” (CV), by the arterialarterial communicants, and by the “cutaneous perforators” (CP), all exhibiting specific regional distribution [1,4,5]. The latest level encompasses direct and indirect small cutaneous perforators of the arterial terminal branches that “pierce from the deep tissue vasculature to the skin surface” [1,5-7]. There have been identified approximately 374 constant CPs vessels ~0.5 mm in diameter in the human body, [1,5-7] including distinct groups located in the inferior limb (Figure 4).
Figure 4: A more detailed representation of main CPs paths in
deep tissue and towards superficial cutaneous layers.
Same notations concerning the type of CP’s collateral
flow distribution:
1=Superficial irrigation to the related skin territory
2=Deep tissue CP connections with correspondent
angiosomal perforators branches in a 3D-distribution
network.
In the initiatory Taylor’s concept, all the 3D tissue blocks that constitute the “angiosomes” are constantly surrounded by the “choke-vessels” and share their flow also through small caliber “collaterals”, adding true “arterial-arterial interconnections” (without changes in calibre), and CPs in a complex compensatory flow system [1-3]. Human body angiosomes have been suggested to correlate to specific clinical boundaries (in deep tissues and at the skin level) that show constant topographic reproducibility [1,3-5]. These clinical and anatomical appearances convey parallel hemodynamic features that depend on each angiosomal perimeter of anastomotic vessels and connect each angiosome with neighboring angiosomes [1,3,7].
The angiosomes are not anatomically indivisible vascular structures [1-5]. They can be gradually subdivided into “final branches” in the vascular tree (before the arteriolar level) [1,4,5,8]. The skin adjacent to the nearest angiosome, receives irrigation from both, tiny terminal branches represented by the CPs, and via the CVs [4,5].
In the last decade, other important findings concerning the skin topographic vasculature were described such as the “perforasome theory” (PT), equally evoked in Taylor et al.’s [4] and Rosen’s parallel work [6]. This theory evokes reproducible clusters of major skin perforators that hold unique vascular territories (called “perforasomes”) [4]. Similar to angiosomes, at the skin level, [4] the perforasomes supply either direct or indirect skin branches and interconnections that arranged in an axial (near the articulations) or a multidirectional (at midsegments) array [4,6]. Conducting similar research, Rosen et al. described “the perforator angiosome” as depending on the main skin CP location [6]. The authors revealed the importance of subcutaneous collateral branching in different patterns of skin perfusion detected by CT-Angiography [6]. These findings appeared to match initial descriptions of skin angiosomes, equally used by Taylor et al. [4,5] as to illustrate the complex cutaneous and “patchy” distribution of CV and CP, in relation to the main angiosomal source artery [4].
According to the AC, six distinct angiosomes (Figures 1-3) and surrounding small choke-vessels, together with specific collaterals, adjacent arterial-arterial interconnections, and terminal CP (Figure 4), were described for foot and lower ankle territories [1,3-8].
Appropriate knowledge of these anatomical structures is probably manifold beneficial for the advised specialist. It affords rescue flow solutions in various ischemic limb conditions, [3,8] a more effective selection for eventual amputation levels and enables better planning of revascularization for optimal tissue regeneration [1,3-5,8].
Clinical aspects: Ischemic foot ulcers gather complex and multifaceted etiological factors, beyond severe hemodynamic impairments. Focusing on their ischemic features alone, there is a huge clinical variability of regional foot flow compensation dictated by countless patterns of collateral flow distribution around the hypoxic tissue zones. Comparable ulcers having same angiosomal location in distinct patients exhibit yet totally different aspects, treatment availabilities, and prognostic, mainly dictated by each individual collateral reserve.
Lower leg and foot angiosomes and appended collaterals
Concerning the lower leg and foot skin and underlying tissue territories, six angiosomes (source arteries and collaterals) were described as follows (Figures 1-3) [1,3-5] .
The posterior tibial artery along its medial calcaneal branch and further via the medial and lateral plantar arteries and defined angiosomes supplies the plantar heel and the entire medial and lateral plantar aspect of the foot and toes [1,3].
The anterior tibial artery, changes into the dorsalis pedis at the dorsum of the foot, supplies its angiosome and provides specific flow to the dorsum of the foot up to the dorsal surface of toes. The anterior tibial artery also nourishes the upper and the anterior peri-malleolar ankle vascularization [1,3].
The peroneal artery supplies flow to a more confined zone of the posterolateral heel through its lateral calcaneal artery angiosome and to the lateral anterior ankle through its anterior perforating branch and correlated angiosome [1,3].
At the upper ankle, additional angiosomes and linked source arteries have been described, such as the anterolateral malleolar branch and related angiosome, adjacent anteromedial malleolar angiosome (both from the anterior tibial artery), corresponding posteromedial malleolar angiosome and associated branches arising from the posterior tibial trunk [1,3-5].
From a clinically applied perspective, the anterior tibial artery affords flow to the anterior ankle and the dorsum of the foot and toes, while the posterior tibial artery nourishes the medial and posterior-medial ankle and heel territories and the entire sole, equally and the plantar angle of the toes (Figures 1-3).
As a source artery, the peroneal trunk supplies the lateral and anterior upper ankle areas and the lateral heel region [3-5].
It should be emphasized that specific skin foot zones expressing complex ischemic wounds, such as the forefoot and hindfoot territories, are mostly supplied by mixed flow arising from two to three neighboring angiosomes [3-5,8-10]. Such cases, including specific hallux necrotic lesions, currently involve more than one foot angiosome and require appropriate multiangiosomal revascularization (by direct angiosomal branches, or via collaterals) [3,8,10].
In daily vascular practice, the AC may be particularly useful for treating “collateral-exhausted” ischemic wounds in diabetic and renal patients [8,10-12]. In these patients, normal “arterialarterial” connections, “CVs”, and the main “CP” may be extensively damaged by more intense and multistage occlusive arterial disease [8,10-13].
Parallel co-morbidities [8,11] such as chronic inflammation, fibrotic scars, local necrosis, regional edema and expanding foot infection, additionally enhance acute thrombosis of small digital and cutaneous perforator collaterals [8,10,11]. Characteristic for chronic limb threatening ischemia (CLTI) condition, specific collateral destruction encountered in diabetic or renal subjects [8,11] differentiates the “angiosome” and the “perforator” vessels endovascular and surgical applications, [4-6] compared to surgical flap interventions in non-ischemic and collateral-fitted patients [4-6,14].
Clinical aspects: Specific foot territories harboring complex ischemic wounds, such as the forefoot and hindfoot regions, are currently supplied by compounded flow from two to three adjacent angiosomes [3-5,8-10]. Such presentations, including hallux and/or toes necrotic lesions, involve more than one foot angiosome and compel appropriate multi-angiosomal reperfusion (by direct source-artery, or by collateral-supported revascularization) [3,8,10].
Main collateral and arterial-arterial connections between adjacent foot angiosomes
An impressive compensatory collateral network interconnecting adjacent foot and ankle angiosomes was documented in previous publications [1,3-5,8,12-14]. Adjacent angiosomes encompass numerous “smallsized” to “large-sized” collaterals and true arterial-arterial interconnections, [3-5] in addition to the small “CVs” [3-5] in a sequential model of perfusion [4,8-10]. Skin associated with each angiosomal territory receives either direct branches from the main source artery [3,4-6] or indirect (terminal) ramifications grouped in specific clusters, or “perforasomes” [4,6]. Numerous “large” (approximately 1 mm diameter) collaterals and interconnections in the foot hold particular importance in supplying adjacent angiosomes during ischemic conditions [3-5,8-10]. These collaterals and interconnections also appear to play a pivotal role in intentional wound-targeted revascularization (WTR) and appropriate tissue regeneration [8,10,12,13]. These vital, large arterial interconnections assemble the foot arches (accounting for 5-9% of anatomical variations), [8,15] metatarsal perforators, anterior or posterior interconnections, and other sizable arterial-arterial branches, such as the dorsal foot-to-plantar or peroneal-toposterior tibial “rescue” heel collaterals [3,5,10,12].
Inside the same design yet with secondary compensatory significance, the “medium-sized” to “small-sized” muscular collateral arteries, the numerous CVs, the CPs, and the arterioles [3-5,8] also contribute to regional limb flow preservation [1,3-5,8]. Regardless of occasional individual variations, the following groups of collaterals and arterial-arterial interconnections were appointed as being important for inter-angiosomal foot flow compensation:
Communication between posterior tibial and peroneal arteries (via medial and lateral calcaneal branches, also via the posterior peroneal branch) [1,3-5] plays an important role in supplying ischemic heel ulcers. These collaterals (if available) equally represent notable flow shifts for targeted hindfoot or heel intentional revascularization [3,8,12,13].
Connections between anterior tibial (dorsalis pedis) and posterior tibial (the plantar arteries) ensure either directly, via the diagonal arteries, or indirectly, via metatarsal perforators (of which the first perforator is the largest interconnection), a substantial supply of blood between the dorsal and plantar foot sectors [1,3-5]. The metatarsal paired anterior and posterior interdigital collaterals provide significant compensation for the forefoot and toes tissue flow preservation and for tarsal/metatarsal reperfusion [3,8,12,13].
Arterial compensation around peri-malleolar ischemic wounds is reinforced by lateral and medial peri-malleolar anastomoses [1,3-5]. The former lateral connections link the peroneal artery (via the anterior perforating branch) with the anterior tibial trunk (via the antero-lateral malleolar branch). The next medial junctions include the medial peri-malleolar network, which shares similar medial malleolar branches from the anterior and posterior tibial arteries [1,3,4]. Both systems are complementary but distinct pathways for blood compensation in the ankle [1,3-5,14].
Interconnections between both plantar arteries (medial and lateral from the posterior tibial artery) and lateral and medial tarsal arteries (via the anterior tibial artery) seem to enable influential compensatory flow [1,3-5,14] for potential plantar ischemic wounds [3,8,10,12].
All these collaterals and true arterial-arterial communications constitute but a small part of the whole natural foot compensatory system against aggressive ischemia [3,8,12].
In this exhaustive “regional view” of peripheral tissue perfusion, albeit more precise than blunt angiographic assessment, [8,12] it appears that not all foot areas may express similar ischemic affliction during CLTI [8,10,12,13].
It has been shown that diabetic and renal CLI patients express serious tissue regeneration impediments and specific infragenicular arterial collateral depletion [8,10-12,16]. A significant decay in individual collateral reserve and CP function was seemingly observed and appeared proportionate to the type and time of ischemic suffering [8,11,16].
Clinical aspects: The most evident ischemic ulcer or necrosis area may not always express the lowest perfusion point in several CLTI feet [8]. The local limb loss of collaterals, [8,11] the “patchy” distribution of remnant CVs and CPs, [6,8] eventual presence of severe neuropathy (associating skin capillary shunting), [11,16] and local sepsis with inflammatory swelling, [11,16,17] may add individual variations in presentations and explain this apparent discrepancy.
Lower leg skin vasculature in light of the angiosome concept
In their initial anatomical report, Taylor and Palmer revealed that the main skin territories in the body receive concomitant direct and indirect small perforator arteries (Figure 4) that extend from deep tissue source vasculature to the skin surface [4,5,18]. The authors focused on the principal anatomical territory as being distinguished from the potential clinical territory of each cutaneous perforator. The perforator anatomical territory, whether called “cutaneous angiosome”, [1,18] or “perforator angiosome”, [4,6,19] (or more condensed “perforasome”), [4,6] encompasses a specific perimeter of anastomotic CV that connects this territory with adjacent skin perforators in all (3D) directions [4,6]. The corresponding perforator clinical territory is wider, it extends beyond the anatomical territory and stretches throughout neighboring perforator zones, particularly if “true” arterial-arterial anastomoses (without changes in CV calibre) are present [3-5]. The limits of perforator clinical territories (Figure 4) are assumed in a dynamic process of dominant/ non-dominant regional perfusion [6,18]. Previous surgery, tissue scarring after repetitive inflammation and individual risk factors (obesity, local ischemia, diabetic neuropathy, collateral septic thrombosis, the vasoactive medication, etc.) may hinder perforator perfusion and local flow compliance [4,6,11,14]. Angiosomal perforators or clusters of perforators are also strongly influenced by each subject’s body weight, local temperature or potential ischemic changes (edema and necrosis), by endogenous neuronal or neuroendocrine substances and by various systemic medications [6,14,18,19]. When defining the 3D angiosomes, including related skin territories, Taylor et al. assumed further gradual subdivision of these vascular structures down to the final prearteriolar terminal branches in the skin and underlying tissues [4,18]. At the skin level, these terminal branches are represented by the CPs (that give rise to characteristic “skin modules” or informally labeled, “cutaneous angiosomes”), and by terminal CVs [4,6,18,19] The authors estimated an average of 4.7 such structures per source artery, [4,5,18] with notable variations in size and number among different angiosomes of the human body [4,18]. In each case, the cutaneous perforator supplied not only the appended skin modules but also a whole tissue block starting from the deep fascia to the epidermal zone [4,5,18]. Apparent discrepancies between the initial angiosomal anatomical description [1] and the clinical skin territories of perforators were appointed in surgical flap applications [3-6,18,19].
These concerns may equally explain parallel controversies in contemporary vascular diagnostic methods targeting CLI revascularization [8,10,20-22].
Clinical aspects: Contemporary publications reveal that using single-method clinical evaluation of the micro-circulation [8,10,16,22-24] either by Laser Doppler, [21,22] by TcPO2, [8,17] or by tissue “oxygen saturation” (StO2) mapping, [10,20] the “source artery’s” precise topography inside the “skin perforator module” distribution [4,6,18] may appear quite difficult to recognize [8,25]. This becomes even more evident during the perioperative assessment of the profoundly distorted collateral vasculature in CLTI, and particularly concerning the neuroischemic diabetic foot [8,10,12,25].
Clinical Research
Our team performed a retrospective review of consecutive CLTI angiographic patterns treated in an eight-year period in our institution. From April 2009 to November 2017, a series of 323 limb-threatening ischemic foot wounds (Rutherford 4-6) [23,24] in 295 patients (71% men), mean age 73.7 years were reviewed. The CLTI characteristics [23] of included population are abridged in Table 1. Main demographic features of this cohort are summarized in Table 2. All TASC II [23] angiographic patterns were defined by preoperative Angio-CT, or Angio-MRI, in association with intraoperative angiography.
| Type of Exam | AP | TP | ABI | TcPO2 | SPP |
|---|---|---|---|---|---|
| CLI features | < 50 mm HG | < 30 mm Hg | < 0.5 | < 40 mm Hg | < 30 mm Hg |
Table 1: Schematic representation of the main levels of arterial division in the inferior limb.
| Patient characteristics data base | All treated Limbs n=323 |
|---|---|
| Age >70 years | 197 (61%) |
| Male gender | 229 (71%) |
| Hypertension | 265 (82%) |
| Diabetes | 196 (60%) |
| Smoking | 145 (45%) |
| Coronary disease | 266 (81%) |
| Cardiac Insufficiency and LVEF < 30% | 94 (29%) |
| Chronic renal insufficiency | 87 (27%) |
| Hypercholesterolemia | 232 (72%) |
| Cerebrovascular disease | 65 (20%) |
| COPD | 100 (31%) |
Table 2: Patient characteristics database.
All data were recorded and analyzed in conformity with correspondent “Levels” of arterial ramifications, [8,25] concerning the “source arteries” and appended collateral distribution (Table 3). For each anatomical level the worse atherosclerotic lesions were detected, then compared with adjacent levels in same CLTI individual pattern. For each ramification level and correspondent branches, the heaviest occlusive angiographic lesions (TASC II “C-D”) [23-25] were documented and defined as “dominant”, in comparison with “associate” atherosclerotic locations (Tables 4 and 5).
| Levels of Perfusion | Type of limb arteries | Arterial segmentation |
|---|---|---|
| Level I. | The originator Arterial and Venous bundles of the Inferior Limb | The Iliac and Common Femoral vessels |
| Level II. | The first rank arterial division: Branches in the Thigh and Calf | The Superficial and Profonda Femoris, The three Tibial Trunks |
| Level III. | The second rank arterial division: Specific tissue sectors branches | The Pedal and Angiosomal branches, The Foot arches, The Large collaterals (around 1 mm) |
| Level IV. | Tissue sectors interconnections | The Medium-sized collaterals (0.5-1 mm), The Small collaterals (< 0.5 mm), The "Choke-Vessels", The skin and muscular Perforators. |
| Level V. | The micro-circulatory arteriolar ramifications | The Arterioles |
| Level VI. | The capillary tier | The Capillaries |
Table 3: Levels of Perfusion.
| Levels of Perfusion (diabetics) | Highest severity/Dominant angiographic lesions (TASC C-D) |
Associated angiographic lesions (severe stenoses >70%, or spotted occlusions) | n (%)Total limbs (n=196) |
|---|---|---|---|
| Level I. | Iliac and Common Femoral vessels | Superficial, Profonda Femoris, +/- Popliteo-Tibial Trunks | 6 (3%) |
| Level II. | Superficial and Profonda Femoris | _ | 14 (7%) |
| Superficial, Profonda Femoris and Popliteal | Tibial arteries | 36 (18%) | |
| Popliteal and Tibial lesions | Pedal arteries | 64 (33%) | |
| Level III. | Distal Tibio -Pedal lesions, and Angiosomal branches | Tibial arteries and Foot arches | 67 (34%) |
| Foot arches and Large collaterals (> 1 mm) | _ | 10 (5%) | |
| Level IV. | The Medium-sized and the Small collaterals (< 1 mm), | Associated evaluation to Levels I-III analysis | 122/196 (62%) |
| Level V. | The Arterioles | Without angiographic analysis | _ |
| Level VI. | The Capillaries | Without angiographic analysis | _ |
Table 4: Levels of diabetic atherosclerotic lesions (n=196).
Results
In a majority of 313 angiographic presentations (97%), chronic total arterial occlusions (TASC II “C-D”) of different lengths (0.5-25 cm) and calcific stages, and at different levels of limb vasculature were demonstrated (Tables 4 and 5). We compared 196 diabetic CLTI presentations versus 127 common CLTI atherosclerotic patterns of occlusive disease at different levels of arterial ramification. Notable differences in the distribution of dominant occlusive lesions were observed (Tables 4 and 5) among groups.
| Levels of Perfusion (non-diabetics) |
Highest severity/Dominant angiographic lesions (TASC C-D) | Associated angiographic lesions (severe stenoses >70%, or spotted occlusions) | n (%) Total limbs (n=127) |
|---|---|---|---|
| Level I. | Iliac and Common Femoral vessels | Superficial, Profonda Femoris, +/- Popliteo-Tibial Trunks. | 13 (10%) |
| Level II. | Superficial and Profonda Femoris | _ | 22 (17%) |
| Superficial, Profonda Femoris and Popliteal | Tibial arteries | 41 (32%) | |
| Popliteal and Tibial lesions | Pedal arteries | 38 (30%) | |
| Level III. | Distal Tibio-Pedal lesions, and Angiosomal branches | Tibial arteries and Foot arches | 6 (5%) |
| Foot arches and Large collaterals (> 1 mm) | _ | 0 | |
| Level IV. | The Medium-sized and the Small collaterals (< 1 mm), | Associated evaluation to Levels I-III analysis | 36/127 (28%) |
| Level V. | The Arterioles | Without angiographic analysis | _ |
| Level VI. | The Capillaries | Without angiographic analysis | _ |
Table 5: Levels of non-diabetic atherosclerotic lesions (n=127).
Specific “Level I” iliac and common femoral lesions (10%), adding “Level II” superficial femoral (17%) also “Level II” femoro-popliteal (32%) dominant occlusive lesions in non-diabetics prevailed against correspondent iliac (3%), superficial femoral (7%), and femoro-popliteal (21%) occlusive lesions in diabetic CLTI subjects.
Conversely, “Level II” distal popliteal and tibial occlusions (33%), “Level III” pedal and angiosomal branches (34%) also “Level III” dominant foot arches occlusions (5%) in diabetic patients, overruled correspondent popliteo-tibial (30%), pedal-angiosomal (5%), and isolated foot arches (0%) lesions in non-diabetics (Tables 4 and 5).
In the same angiographic analysis yet owning retarded contrast filling evaluation, the medium to small-sized collateral assessment (Level IV), showed striking differences in the severity and extent of collateral destruction (62% in diabetics, against 28% in nondiabetics).
Discussion
Following previous clinical observation [8,25] and according to the present analysis, several ranks of arterial and collateral division of the inferior limb vasculature can be stipulated. These “Levels” or degrees of harmonious and graduated branching may harbor specific atherosclerotic affectation, concordant with main CLTI initiatory pathologies [8,23-25].
Specific levels of perfusion of the inferior limb vasculature
It has been already showed that the human body possesses a characteristic collateral system that assists blood supply between neighboring angiosomes [1-4]. This remarkable compensatory network continuously provides coordinated and dynamic changes in regional perfusion according to various endogenous and exogenous factors [3-6,8,25].
Every arterial trunk progressively divides into inferior graduated degrees of segmentation to create a larger cross-sectional surface toward peripheral tissues [1,15,26]. Moreover, each division branch is progressively thinner than its parent trunk [15,26]. For every arterial bifurcation, the added sectional area of the two secondary branches appears to be superior to that of the genuine vessel [15,26,27]. Angiosomes, associated collaterals, CPs, and CVs together assemble a harmonious “pyramid of gradual flow distribution” toward specific 3D peripheral building blocks of tissue [4,8,15,25,27].
Following previous perioperative 2D angiographic observations of our team, [10,25] several Levels of decreasing caliber arterial ramifications were described and summarized in Table 3.
Level I of perfusion encompasses the original arterio-venous bundles of blood supply of the inferior limb (i.e., iliac and common femoral).
Level II includes “first rank” division branches in the thigh and calf (i.e., superficial and profunda femoris and the three tibial trunks).
Level III features characteristic terminal branches for specific 3D sectors in the skin and underlying tissues in the leg and foot (“second rank” division branches).
This Level III also includes the angiosomal “source” arterial branches, the large collaterals (around 1 mm), the arterial-arterial interconnections, several corresponding diameter metatarsal branches, some of CPs and the foot arches [8,10].
Level III offers specific interventional interest for performing topographic WTR [8,10,25].
Level IV reunites the medium and small collaterals (inferior to 1 mm), including most cutaneous and muscular perforators (CP) and the abovementioned CVs [8].
In practice, Levels III and IV encompass the majority of the superficial CVs and CPs also the deep direct and indirect perforator branches from deep tissue that surface at the skin level [4].
Succeeding microcirculatory ranks unfold the next two levels, inaccessible by sole angiographic exploration.
Level V that contains the arterioles and the Level VI, that gathers the capillary tier (around eight micrometers-diameter vessel) [15,27].
This latter, comprises “several millions of tiny microscopic conduits” in the whole body.
A previous reported indicated that this level can extend to “an estimated length of approximately 60,000 miles” [15].
An alternative and more common anatomical stratification was also described in contemporary literature. This binary classification basically distinguishes the macrocirculatory (Levels I to IV) from the «microcirculatory» (Levels V and VI) ranks for limb perfusion [8,15,25-27].
By joining these levels, the large- to small-diameter arteries are connected to adjacent arterioles and dynamically contribute to a continuous «pacing system» for regional tissue perfusion [8,24-28].
Following iterative ischemic threats, local sepsis and inflammation, or distally extended atherosclerotic and thrombotic disease, basic compensatory conditions may be surpassed [8,10-12]. The devastating hemodynamic effects of CLTI ultimately depend on each lasting collateral reserve in every individual pattern of compensation (Levels II-V).
As focused in the “research” section, preferential location of genuine atherosclerotic CLTI disease in Levels I-II, can be distinguished from characteristic Levels III-IV arterial CLTI affectation evinced in diabetic patients [8,10-12].
Clinical aspects
Specific vascular evaluation focusing on individual “Levels” of arterial division can be useful for the advised clinician. In young and non-diabetic patients with unaltered source arteries, foot arches, arterial-arterial interconnections, and patent large to small diameter collaterals, CVs or CPs (Levels III, and IV), eventual ischemic injuries successfully activate this high-performance inter-angiosomal rescue system [3-5,8,10,26].
In these CLTI cases, topographic WTR, or direct revascularization (DR) via “source arteries” or collaterals (Levels III-IV), may stir only discreet clinical improvements compared to indirect revascularization (IR) [8,22,28,29].
In opposite presentations featuring a severely injured collateral reserve (Levels III to V), nonselective revascularization (via neighboring angiosomes, CVs, and CPs) warrants only modest compensatory flow pressure between adjacent angiosomes [3,8,12,13,25]. In these patients, appropriate WTR, or DR (if technically achievable) may yield improved hemodynamic conditions for quicker tissue recovery [8,12,13,25].
Anatomical variants, acquired changes and implications for lower leg arterial flow
Native variants of arterial perfusion: Following meticulous anatomical studies over the last decades, several innate foot arterial variants have been reported and indexed, mainly concerning “Level III” of limb flow distribution [8,30].
Consistent with a recent meta-analysis by Kropman et al. assembling 7671 cases, [31] and with other parallel “in vivo” angiographic data, [9,19] native atypical leg arteries appeared to be observed in approximately 7.9-10% of individuals in the general population [9,30,31].
Among these variations, posterior tibial artery hypoplastic, aplastic or high origins were encountered in 3.3% of cases, whereas the anterior tibial artery absence was documented in 1.5% of instances [30].
A highly located emergence of the anterior tibial artery or unusual tibial trifurcations were reported in 5.6-6.2% of cases, while uncommon dorsalis pedis origins was assigned in 4.3-6% of presentations [30,31].Abnormal first dorsal metatarsal artery origin and related first toe vascularization were described in 8.1% of patients, concomitant variations of the arcuate artery in 5%, and atypical plantar arch and plantar arteries trajectories in 5% [25,30,31].
Particular knowledge of these possible anatomical variants may help the advising specialist while planning wound-targeted, or angiosome-guided revascularization [8,31].
Detection of eventual popliteal variation on the treated extremity (i.e., the high emergence of the anterior tibial artery) may alert on 21% of possible homolateral foot arteries abnormalities and near to 48% of contralateral inferior limb vascular variants [30,31]. The presence of native arterial variants is generally conceived inside same ranks of arterial dichotomy and globally do not seem to alter the above mentioned six level scaffolding for arterial distribution [25].
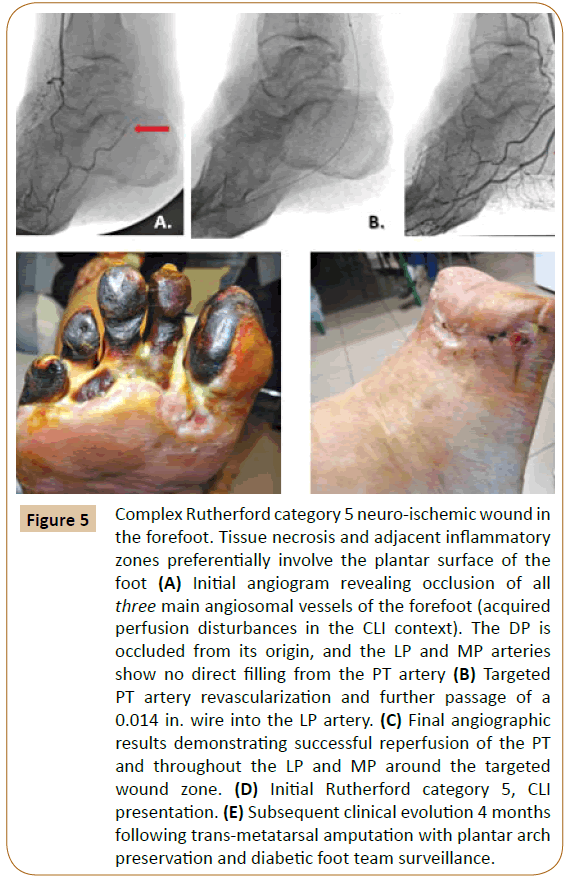
Developed flow changes: Concomitant and more frequent acquired arterial course disturbances have also been cited in CLI presentations [8,22-24]. A majority of these vascular irregularities were found in patients with specific diabetic “neuro-ischemic” feet (Figure 5), or with concomitant uremic syndromes [8,22-25].
Figure 5: Complex Rutherford category 5 neuro-ischemic wound in the forefoot. Tissue necrosis and adjacent inflammatory zones preferentially involve the plantar surface of the foot (A) Initial angiogram revealing occlusion of all three main angiosomal vessels of the forefoot (acquired perfusion disturbances in the CLI context). The DP is occluded from its origin, and the LP and MP arteries show no direct filling from the PT artery (B) Targeted PT artery revascularization and further passage of a 0.014 in. wire into the LP artery. (C) Final angiographic results demonstrating successful reperfusion of the PT and throughout the LP and MP around the targeted wound zone. (D) Initial Rutherford category 5, CLI presentation. (E) Subsequent clinical evolution 4 months following trans-metatarsal amputation with plantar arch preservation and diabetic foot team surveillance.
The above-mentioned research data confirm this assertion particularly for the CLTI diabetic subjects. The dominant belowthe- knee presentations (equivalent to Levels III-IV) have been reported in more than 75% of diabetic CLI patients [23-25,32,33].
Distal limb collateral architecture can be highly restructured in such severely distorted hemodynamic patterns [1,32]. Classical angiosomal orientation itself may become challenging in these situations [8,16,23,32] (Figure 5).
Regardless of the native, or the acquired origin in arterial flow affectations, specific CLTI pathologies inflict peculiar changes in the whole arterial system of the inferior limb.
Clinical aspects: Taking in account possible innate or acquired changes, increasing clinical evidence currently recommends precise angiographic support in planning eventual topographic revascularization [8,12,34]. The gathering of macro-and microcirculatory assessment in each individual CLTI presentation, scheduled for all six “Levels of arterial perfusion”, appears to be useful for improved flow outcome [8,2]. To dominant Levels I-II “common” atherosclerotic occlusive disease, specific Levels III-IV severe CTOs are opposed in the diabetic CLTI patients [8,10,12,30,31].
Limitations of the Study
The present analysis discloses some inherent limitations. The majority of clinical observation was based on 2D Digital Subtraction Angiography (DSA) equipment that presents specific impediments in thorough distal foot collateral estimation. This appears particularly true in slow flow and collateral deprived CLTI subjects, with only relative estimation of “dormant”, or “latent” Level IV collaterals, distal to long and highly calcific remote arterial occlusions.
It should be also noted that in about 29% of cases (Table 2) the presence of severe cardiac insufficiency (LVEF < 30%) added complementary hemodynamic hurdles in objective small branches appraisal of foot perfusion.
From a micro-circulatory point of view this study cannot provide more detailed information concerning peculiar CLTI changes at the arteriolar and at the capillary Levels V, and VI of foot perfusion. These vascular ranks still remain inappreciable by sole DSA imaging modalities [35].
Finally, the notion of “desert foot” (severe collateral loss of Levels III, and VI) for some CLTI presentations proved real difficulties in recognition during most initiatory groin DSA injections. As mentioned by Diehm, [36] “Digital subtraction angiography still represents a purely luminographic imaging modality that is impacted by individual hemodynamic situation of the patient and the position of the sheath” [36]. Exclusive flow-dependent contrast imaging technology can only partially reflect remote microcirculatory changes of the severely affected ischemic foot [37].
Conclusion
Inferior limb vasculature encompasses a harmonious arrangement of specific 3D “tissue-oriented” arterial axes, including the angiosome-directed source arteries. These branches are coupled to a vast collateral compensatory system (CVs, CPs, and associated arterial-arterial interconnections). Following the same balanced vascular distribution, several Levels of inferior limb arterial ramification (Levels I-VI) can be described on angiographic analysis. To specific Levels I-II bear atherosclerotic occlusive disease, characteristic Levels III-IV topographic chronic occlusions are opposed in diabetic CLTI patients.
References
- Taylor GI, Palmer JH (1987) The vascular territories (angiosomes) of the body: Experimental study and clinical applications. Br J Plast Surg 40: 113-141.
- Taylor GI, Minabe T (1992) The angiosomes of the mammals and other vertebrates. Plast Reconstr Surg 89: 181-215.
- Attinger CE, Evans KK, Bulan E, Blume P, Cooper P (2006) Angiosomes of the foot and ankle and clinical implications for limb salvage: reconstruction incisions and revascularization. Plast Reconstr Surg 117: 261S-293S.
- Taylor GI, Corlett RJ, Shymal CD, Ashton MW (2011) The anatomical (angiosome) and clinical territories of cutaneous perforating arteries: development of the concept and designing safe flaps. Plast Reconstr Surg 127: 1447-1459.
- Taylor GI, Pan WR (1998) Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg 102: 599-616.
- Rozen WM, Ashton MW, Le Roux CM, Pan WR, Corlett RJ (2010) The perforator angiosome: A new concept in the design of deep inferior epigastric artery perforator flaps for breast reconstruction. Microsurgery 30: 1-7.
- Hong MK, Pan WR, Wallace d, Ashton MW, Taylor GI (2008) The angiosome territories of the spinal cord: exploring the issue of preoperative spinal angiography. Laboratory investigation. J Neurosurg Spine 4: 352-364.
- Alexandrescu VA, Defraigne JO (2018) Angiosome system and principle Chapter 77: in Lanzer P. Textbook of Catheter-Based Cardiovascular Interventions. Springer 1344-1358.
- McGregor AD (1992) The angiosome: an in vivo study by fluorescein angiography. Br J Plast Surg. 45: 219-221.
- Alexandrescu VA, London V (2015) Angiosomes: the cutaneous and arterial evaluation in CLI patients. (Chapter 5), in: « Critical Limb Ischemia: CLI diagnosis and interventions: Mustapha JA editor. Chicago, HMP 71-88.
- O’Neal LW. Surgical pathology of the foot and clinicopathologic correlations. In: Bowker JH, Pfeifer MA. Levin, O’Neal’s editors. The Diabetic Foot, 7th edn., Mosby Elsevier, Philadelphia. 2007: 367-401.
- Zheng XT, Zeng RC, Huang JY, Pan LM, Su X, et al. (2016) The use of the angiosome concept for treating infrapopliteal critical limb ischemia through interventional therapy and determining the clinical significance of collateral vessels. Ann Vasc Surg 32: 41-49.
- Osawa S, Terashi H, Tsuji Y, Kitano I, Sugimoto K (2013) Importance of the six angiosomes concept through arterial-arterial connections in CLI. Int Angiol 32: 375-385.
- Crawford ME (2006) Flap classification and survival factors. In: Dockery GL Crawford ME editors. “Lower Extremity Soft Tissue and Cutaneous Plastic Surgery.” pp: 97-104.
- Williams PL, Warwick R, Dyson M, Bannister LH (1996) Angiology blood vessels. In: Gray’s Anatomy. 38th Ed. London: Churchill Livingstone 682-694.
- Marso SP, Hiatt WR (2006) Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol 47: 921-929.
- Jörneskog G (2012) Why critical limb ischemia criteria are not applicable to diabetic foot and what the consequences are. Scan J Surg 101: 114-118.
- Taylor GI (2003) The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg 30: 331-342.
- Rozen WM, Grinsell D, Koshima I, Ashton MW (2010) Dominance between angiosome and perforator territories: a new anatomical model for the design of perforator flaps. J Reconstr Microsurg 26: 539-545.
- Kagaya Y, Ohura H, Suga H, Eto H, Takushima A, et al. (2014) Real angiosome assessment from peripheral tissue perfusion using tissue oxygen saturation foot mapping in patients with critical limb ischemia. Eur J Vasc Endovasc Surg 47: 433-441.
- Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, et al. (2008) Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs: comparison with other noninvasive diagnostic methods. J Vasc Surg 47: 318-323.
- Kawarada O, Yokoi Y, Higashimori A, Waratani N, Fujihara M, et al. (2011) Assessment of macro- and microcirculation in contemporary critical limb ischemia. Catheter Cardiovasc Interv 78: 1051-8.
- Elsayed S, Clavijo LC (2015) Critical limb ischemia. Cardiol Clin 33: 37-47.
- Chin JA, Sumpio BE (2014) New advances in limb salvage. Surg Technol Int 25: 212-216.
- Alexandrescu VA (2012) Angiosomes applications in Critical Limb Ischemia: in search for relevance. Torino. Minerva Medica.
- Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, et al. (2010) Marvels, mysteries and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation 17: 3-20.
- Schaper W (2009) Collateral circulation, past and present. Basic Res Cardiol 104: 5-21.
- Rashid H, Slim H, Zayed H, Huang DY, Wilkins CJ, et al. (2013) The impact of arterial pedal arch quality and angiosome revascularization on foot tissue loss healing and infrapopliteal bypass outcome. J Vasc Surg 57: 1219-1226.
- Azuma N, Uchida H, Kokubo T, Koya A, Akasaka N, et al. (2012) Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery? Eur J Vasc Endovasc Surg 43: 322-328.
- Yamada T, Gloviczki P, Bower TC, Naessens JM, Carmichael SW, et al. (1993) Variations of the arterial anatomy of the foot. Am J Surg 166: 130-135.
- Kropman RH, Kiela G, Moll FL, de Vries JP (2011) Variations in the anatomy of the popliteal artery and its side branches. Vasc Endovasc Surg 45: 536-540.
- Alexandrescu VA, Triffaux F (2016) Ischemic ulcer healing: does appropriate flow reconstruction stand for all that we need? In “Wound healing: new insights into ancient challenges” Ed. Intech Publ 251-282.
- Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, et al. (2016) The management of the diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 63: 3S-21S.
- Waltenberg J (2001) Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res 49: 554-560.
- Alexandrescu VA (2018) Contributions of the angiosome concept in the management of the ischemic diabetic foot. PhD Thesis University of Liège, Ed. Thomas & Chabot 14-55.
- Diehm N (2015) Commentary: Intra-arterial digital substraction angiography: what you see is not always what you get. J Endovasc Ther 22: 252-253.
- Vallabhaneni R, Kalbaugh CA, Kouri A, Farber MA, Marston WA, et al. (2016) Current accepted hemodynamic criteria for critical limb ischemia do not accurately stratify patients at high risk for limb loss. J Vasc Surg 63: 105-113.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences