Evaluation of Femoro-Popliteal Angioplasties with the Need for Retrograde Approach in a Twin Center Series of 26 Consecutive Cases
Morin Julien, Lakhlifi Emilie, Midy Dominique, Poirier Mathieu, Caradu Caroline and Ducasse Eric
DOI10.21767/2573-4482.100029
Morin Julien1, Lakhlifi Emilie2, Midy Dominique1, Poirier Mathieu2, Caradu Caroline1 and Ducasse Eric1*
1Unit of Vascular Surgery, Universite de Bordeaux, Bordeaux, France
2Unit of Vascular Surgery, CH Layne, Mont de Marsan, France
- *Corresponding Author:
- Ducasse Eric
Department of Vascular Surgery, Tripod Hospital, Amelie Raba-Leon Square, 33076 Bordeaux, France
Tel: 33556795525
E-mail: eric.ducasse@chu-bordeaux.fr
Received Date: October 25, 2016; Accepted Date: November 11, 2016; Published Date: November 24, 2016
Citation: Julien M, Emilie L, Dominique M, et al. Evaluation of Femoro-Popliteal Angioplasties with the Need for Retrograde Approach in a Twin Center Series of 26 Consecutive Cases. J Vasc Endovasc Surg. 2016, 1:4. doi: 10.21767/2573-4482.100029
Abstract
Objectives: To evaluate the feasibility of percutaneous angioplasty using the Sub-intimal Arterial Flossing with Anterograde-Retrograde Intervention (SAFARI) technique in case of femoro-popliteal occlusion.
Methods: This twin centers study included, from November 2012 till April 2015, all the attempts to carry out a femoro-popliteal angioplasty by SAFARI technique after anterograde failure.
Results: Twenty-six patients were included (10 [38.5%] diabetics, mean age 67 ± 12 years). Twelve (46.2%) suffered from claudication and 14 (53.8%) from Critical Limb Ischemia (CLI). Mean lesion length was 175.8 ± 100.3 mm, with 4 (15.4%) Trans-Atlantic Society Consensus (TASC) C and 18 TASC D lesions (69.2%). Technical success was 84.6% (22 patients, 20 [76.9%] popliteal punctures), including 3 (11.5%) “rendezvous” techniques. Mean number of stents/patient was 1.5 ± 1.1 (diameter of 6.1 ± 0.3 mm, 196.0 ± 116.1 mm long) with systematic predilation (4.5 ± 1.1 mm in diameter, 116.8 ± 38.3 mm long balloons). Thirteen patients (50.0%) underwent simultaneous additional procedures. One hematoma (4.2%) occurred at the distal puncture site, without the need for surgery. Mean follow-up was 19.5 ± 12.4 months with a patency rate of 90.0% at 6 months and 50.8% at 12, 24 and 36 months, without redo surgery on target arteries in 100.0% of the cases at 6 months and 84.4% at 12, 24 and 36 months. At latest follow-up, 3 patients suffered from claudication (15.0%) and 6 were in CLI (30.0%). Four of 12 CLI patients were completely healed (33.3%) and 17 (85.0%) were clinically improved. Limb salvage rate was 96.2%. Estimated overall survival rate is 95.7% at 6 months, 90.6% at 12 months, 67.1% at 24 months and 40.3% at 36 months.
Conclusion: The SAFARI technique is a minimally invasive approach increasing per-operative technical success of femoro-popliteal percutaneous angioplasties, without increasing the rate of local complications or influencing patency in the short-run.
Keywords
Retrograde; Superficial Femoral Artery (SFA); Wound healing; Patency; Chronic total occlusion
Introduction
Since the rise of percutaneous femoral angioplasty, the proportion of endovascular revascularization indications has been increasing at the expense of conventional open surgery. Indeed, according to the Trans-Atlantic Inter-Society Consensus (TASC) II classification published in 2007, an endovascular first approach was recommended for TASC A and B lesions [1]. However, TASC C and D lesions, described as belonging to conventional treatment with bypass surgery at the time, are becoming more accessible to endovascular techniques thanks to new devices, making percutaneous femoral angioplasty a therapeutic option whatever the TASC classification [2]. Since, patients with TASC C and D lesions are frequently high-risk surgical bypass patients and have a considerably shorter life expectancy than patients with TASC A and B lesions, endovascular therapy is more and more chosen to improve distal perfusion in order to obtain limb salvage and improve quality of life.
Nonetheless, the primary success rate following a conventional anterograde approach remains limited to 75 to 87%, particularly because of the difficulties of intra-luminal reentry in case of subintimal recanalization through Chronic Total Occlusions (CTOs) of the Superficial Femoral Artery (SFA) [3-5]. Moreover, a re-entry too distal could extend the lengths of sub-intimal space and lead to decreased patency by increasing the length of angioplasty and stenting, or clinical deterioration by increasing the ischemia due to disruption of vital collateral vessels with the consequent risk to lose a surgical option [6-8].
An alternative to enhance the re-entry success rate via an anterograde only approach is the use of controlled re-entry devices but their elevated cost has limited widespread adoption. Moreover, this modality does not address the problem of limited distal target artery available for re-entry [9,10]. To help overcome some of these limitations, another technical possibility is to use the retrograde approach, first reported by Tonnesen et al. [11] and initially performed with patients in a prone position [12]. Then, a double approach via the femoral and popliteal or tibial arteries, named Sub-arterial Intimal Flossing with Anterograde-Retrograde Intervention (SAFARI) technique, was described by Spinosa et al., to help recanalize CTOs [13]. It has gained a renewed interest in the event of recanalization failure by anterograde approach, by offering the possibility of limb salvage in patients ineligible for bypass surgery [11] but has been associated with complications such as dissections, arterial ruptures, arteriovenous fistula, false aneurysms, bleeding, and hematomas, with the risk of inadvertent vessel damage, which is critically important if the punctured artery is the single remaining runoff vessel [14,15].
Furthermore, to avoid changing the patient’s position, popliteal puncture in the supine position and the use of lower-diameter puncture instruments, such as 3 Fr sheaths or micro catheters, have been reported, and it has since gained popularity [16-18].
The objective of this study was to evaluate the feasibility of percutaneous angioplasty using the SAFARI technique in case of femoro-popliteal occlusion, as well as the clinical outcomes and the patency of the revascularization. The secondary endpoint was to detect specific complications of the retrograde approach.
Methods
Study description
From November 2012 to April 2015, all the attempts to carry out a femoro-popliteal angioplasty by SAFARI technique performed by 4 operators in 2 centers (CHU of Bordeaux and CH of Montde- Marsan) were identified and included in a database updated prospectively. Inclusion criteria were represented by the need for femoro-popliteal angioplasty in the context of Peripheral Arterial Disease (PAD) stage 3 to 6 of the classification of Rutherford, with TASC A to D lesions, in case of crossing or reentry failure by anterograde approach. All patients in whom retrograde puncture was attempted were included in the study. Exclusion criteria were represented by successful recanalization with a sole anterograde approach.
This was a retrospective observational study assessing the results of the SAFARI technique for the revascularization of femoro-popliteal lesions. Pre-operative demographic, clinical and anatomical characteristics were collected as well as peroperative and 30-day/in-hospital morbidity and mortality. During follow-up, patency rate, re-intervention-free survival and overall survival were recorded. Rutherford class evolution as well as healing process, for patients Rutherford class 5 or 6 at inclusion, were also analyzed.
Endovascular reconstruction technique
General anesthesia was proposed from the outset in both centers for all patients requiring revascularization (Figure 1). All the procedures were performed in the operating room under general anesthesia and general heparinization at 0.5 mg/kg. The operative field always included the two scarpas and the entire lower limb to be treated.
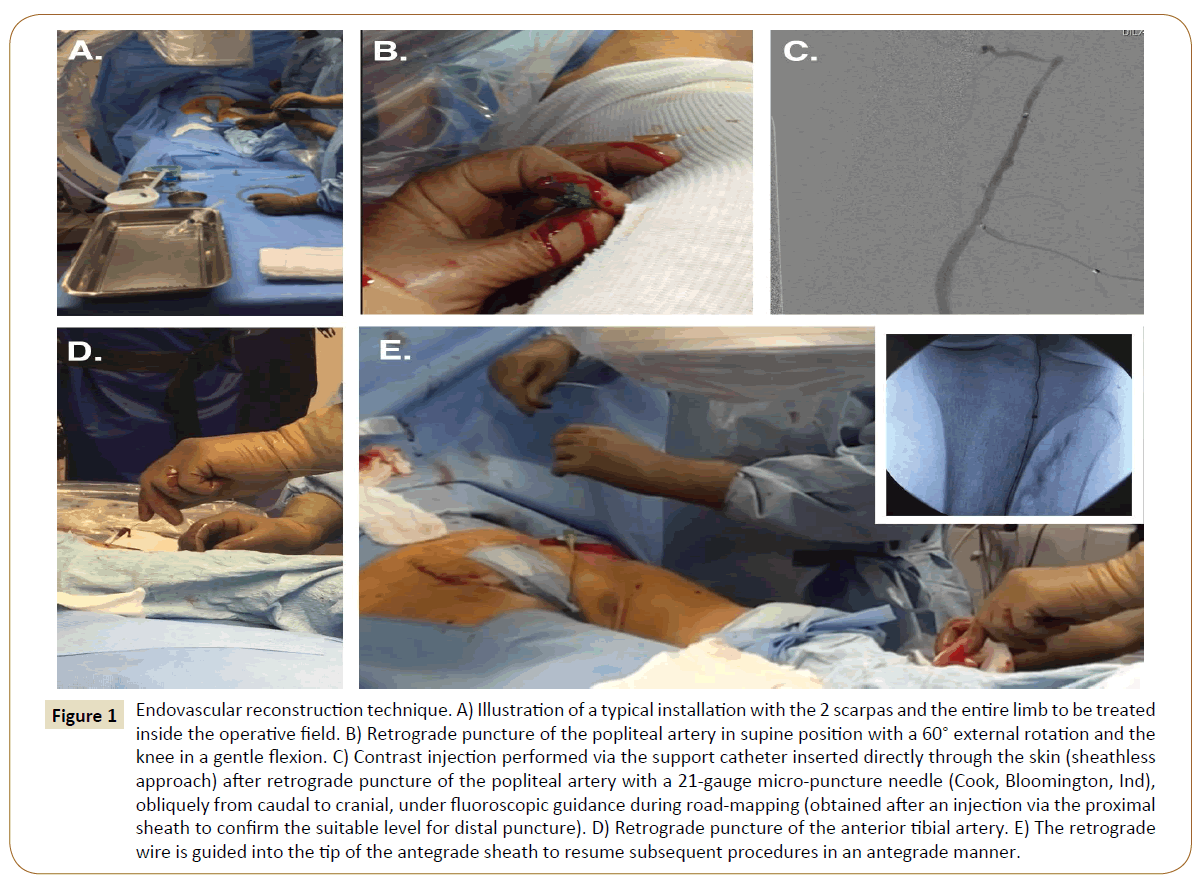
Figure 1: Endovascular reconstruction technique. A) Illustration of a typical installation with the 2 scarpas and the entire limb to be treated inside the operative field. B) Retrograde puncture of the popliteal artery in supine position with a 60° external rotation and the knee in a gentle flexion. C) Contrast injection performed via the support catheter inserted directly through the skin (sheathless approach) after retrograde puncture of the popliteal artery with a 21-gauge micro-puncture needle (Cook, Bloomington, Ind), obliquely from caudal to cranial, under fluoroscopic guidance during road-mapping (obtained after an injection via the proximal sheath to confirm the suitable level for distal puncture). D) Retrograde puncture of the anterior tibial artery. E) The retrograde wire is guided into the tip of the antegrade sheath to resume subsequent procedures in an antegrade manner.
The procedures were performed via an antegrade approach, which was either ipsilateral via the common femoral artery or contralateral via a crossover, depending on the length of SFA stump, which had to measure at least 2 cm for ipsilateral puncture. This step was performed by percutaneous puncture, using 4-6 Fr sheaths, an hydrophilic Terumo® 0.035-inch guidewire (Terumo Europe, Leuven, Belgium) and a Trailblazer® 0.035-inch support catheter (EV3 Europe SAS, Courbevoie, France).
If unsuccessful, a retrograde puncture was then carried out. The patient remained supine, with the lower extremity in a 60° external rotation and the knee in a gentle flexion. An angiogram via the proximal sheath was performed to confirm the suitable level for distal puncture. The popliteal artery was our first choice strategy if eligible, if not, the anterior or posterior tibial arteries were considered. Puncture was performed with a 7 cm 21-gauge micro-puncture needle (Cook, Bloomington, Ind), obliquely from caudal to cranial. The C-arm was adjusted for precise alignment with the axis of the puncture needle on a 90° projection. The puncture was performed on the infra-condylar plane with a roadmap technique under fluoroscopic guidance. When access was obtained, a 0.018-inch V-18 guidewire (Boston Scientific, Natick, and Mass) was inserted, the needle was pulled out, and a support catheter was inserted (Trailblazer® [EV3 Europe SAS, Courbevoie, France] or Seeker® [Bard France SAS, Val-de-Marne, France]). No sheath was adopted.
Usually, the retrograde wire could easily cross the proximal subintimal channel created during the anterograde approach and re-enter into the true lumen. If the wire was not able to cross the lesion, a “rendezvous” technique, also known as « controlled antegrade and retrograde sub-intimal tracking » (CART), was used: 2 small balloons were inserted simultaneously from the antegrade and retrograde directions into the occlusion and placed with a distance between their tips of no more than 5 mm [19,20]. The wires were pulled back inside the balloons and a short inflation was performed to disrupt arteriosclerotic material or the dissection membrane separating the balloons from each other. The balloons were then pulled back and wire passage was attempted again from both directions. For this purpose, the balloons employed were Savvy® (Cordis Corporation, Wilmington New Castle, Delaware, USA), Rival® (Bard France SAS, Val-de-Marne, France) or Passeo® balloons (Biotronik SE & Co., Berlin, Germany). Once across the occluded vessel segment with the retrograde wire, it was guided into the tip of the anterograde sheath to resume subsequent procedures in an anterograde manner. Balloon angioplasty was performed from the anterograde approach with a Power Flex Pro® balloon (Cordis Corporation, Wilmington new Castle, Delaware, USA).
In case of residual stenosis ≥30% or flow-limiting dissection despite prolonged angioplasty (≥180 s), one or more selfexpanding stents were implanted [21]. The choice of the stent was left to the preference of the operator between Lifestent® or Luminexx® (Bard France SAS, Val-de-Marne, France), SMART® Flex (Cordis Corporation, Wilmington New Castle, Delaware, USA), Astron Pulsar® (Biotronik SE & Co., Berlin, Germany), Complete SE® (Medtronik, Dublin, Leinster, Ireland) and Heliflex® (Hexacath, Rueil Malmaison, France). In case of ostial lesions of the SFA a short balloon expandable stent (Dynamic® [Biotronik SE & Co., Berlin, Germany]) was used for better precision deployment to avoid coverage of the deep femoral artery. Postdilation of the stents was performed routinely. At the end of the procedure, prolonged inflation was performed at the location of the retrograde puncture and in the meantime, gentle manual compression was maintained until hemostasis was achieved. The balloon was then deflated, and the patency of the limb was checked by angiography from the anterograde approach. Finally, the anterograde sheath was removed and hemostasis was achieved using percutaneous closure devices (StarClose® [Abbott Vascular, Gustave Eiffel Place, Rungis, France] or Exoseal® [Cordis Corporation, Wilmington New Castle, Delaware, USA STATES]), or by prolonged manual compression.
Operative data were collected, including operative and fluoroscopy times, Kerma-area product, technical success, implanted devices and the length of treated lesions.
Follow-up protocol
All patients were followed by the 4 operators and a wound healing center. The clinical and duplex-ultrasound data were collected prospectively at 1, 6 and 12 months and yearly thereafter. When the patient was a stage 5 or 6 according to the classification of Rutherford, an additional clinical examination was scheduled on the 3rd postoperative month. All wounds were reproducibly measured by the wound healing center with surface data given in cm2. In case of complete healing, the healing time was calculated from the date of the procedure to the date of the clinical examination objectifying this healing.
Statistical analysis
Statistical analyses were performed using GraphPad Prism v6.0 (GraphPad Inc, San Diego, Calif). Kaplan-Meier analyses were used to assess the survival, reintervention-free survival and patency rates at follow-up. Categorical data are reported with absolute numbers and percentage of prevalence (%) and continuous variables with means ± standard deviation. Student t-tests and Chi-square tests were used for parametric data since all statistical distributions were normal according to the Shapiro- Wilk normality test. All tests were two-sided with a significance level <0.05.
Results
Patients
Twenty-six consecutive patients were included over the two centers during the study period. Twenty-one procedures were performed at the CH of Mont-de-Marsan and 5 at the CHU of Bordeaux. The demographics are detailed in Table 1.
| Variables | Patients(N=26) |
|---|---|
| Operative time (min) | 212.6 ± 66.1 |
| Fluoroscopy time (min) | 48.9 ± 15.0 |
| Kerma-area product (cGy/cm2) | 5656.0 ± 2726.7 |
| Technical success | 22 (84.6) |
| Localization of the retrograde puncture | |
| Popliteal | 20 (76.9) |
| Anterior tibial artery | 5 (19.2) |
| Posterior tibial artery | 1 (3.8) |
| “Rendezvous” technique | 3 (11.5) |
| Mean balloon’s length (mm) | 116.8 ± 38.3 |
| Mean balloon’s diameter (mm) | 4.5 ± 1.1 |
| Balloon angioplasty solely | 2 (7.7) |
| Mean stent’s length (mm) | 196.0 ± 116.1 |
| Mean stent’s diameter (mm) | 6.1± 0.3 |
| Mean number of stent/patient | 1.5 ± 1.1 |
| Associated procedures | |
| Femoral thromboendarterectomy | 3 (11.5) |
| Iliac angioplasty | 4 (15.4) |
| Downstream angioplasty | 6 (23.1) |
Table 1: Demographic characteristics. TASC (Trans-Atlantic Society Consensus), ASA (American Society of Anesthesiologists).
The majority of patients were male with a sex ratio of 3.3. Mean age was 67 ± 12 years. Twelve (46.2%) suffered from intermittent claudication (Rutherford class 3) and 14 (53.8%, 2 Rutherford class 4, 4 Rutherford class 5 and 8 Rutherford class 6) from Critical Limb Ischemia (CLI). Mean lesion length was 175.8 ± 100.3 mm, with 4 (15.4%) TASC C and 18 TASC D lesions (69.2%). Twenty-one lesions (80.8%) were ≥100 mm long.
Endovascular reconstruction
Mean operative time was 212.6 ± 66.1 min with a mean fluoroscopy time of 48.9 ± 15.0 min and a Kerma area product of 5656.0 ± 2726.7 cGy/cm2. Technical success rate was 84.6% (22 patients). There were 20 (76.9%) popliteal punctures, 5 (19.2%) anterior tibial and 1 (3.8) posterior tibial, including 3 (11.5%) “rendezvous” techniques.
Systematic pre dilation was carried out with balloons of 116.8 ± 38.3 mm in length and 4.5 ± 1.1 mm in diameter. Mean number of stents/patient was 1.5 ± 1.1, with a length of 196.0 ± 116.1 mm and a diameter of 6.1± 0.3 mm. Two re-canalizations were not stented because of an optimal outcome of primary angioplasty.
Thirteen patients (50.0%) underwent additional procedures simultaneous to the femoro-popliteal revascularization (4 iliac angioplasties [15.4%], 3 femoral thromboendarterectomy [11.5%], 6 downstream angioplasties [23.1%]). Operating data are reported in Table 2. There was one elective minor (toe) amputation during the initial procedure (3.8%).
| Variables | Patients (N=26) |
|---|---|
| Operative time (min) | 212.6 ± 66.1 |
| Fluoroscopy time (min) | 48.9 ± 15.0 |
| Kerma-area product (cGy/cm2) | 5656.0 ± 2726.7 |
| Technical success | 22 (84.6) |
| Localization of the retrograde puncture | |
| Popliteal | 20 (76.9) |
| Anterior tibial artery | 5 (19.2) |
| Posterior tibial artery | 1 (3.8) |
| “Rendezvous” technique | 3 (11.5) |
| Mean balloon’s length (mm) | 116.8 ± 38.3 |
| Mean balloon’s diameter (mm) | 4.5 ± 1.1 |
| Balloon angioplasty solely | 2 (7.7) |
| Mean stent’s length (mm) | 196.0 ± 116.1 |
| Mean stent’s diameter (mm) | 6.1± 0.3 |
| Mean number of stent/patient | 1.5 ± 1.1 |
| Associated procedures | |
| Femoral thromboendarterectomy | 3 (11.5) |
| Iliac angioplasty | 4 (15.4) |
| Downstream angioplasty | 6 (23.1) |
Table 2: Endovascular reconstruction.
Endovascular failures
There were 4 recanalization failures by SAFARI technique on the entire series (15.4%). Two of the patients underwent femoropopliteal bypass surgery in reverse saphenous vein during the same procedure time. Regarding the other two patients, the first was 81 years old and classified American Society of Anesthesiologists (ASA) 3 and Rutherford class 5. Monitoring of the wound by the wound healing center showed a positive evolution despite incomplete revascularization. Due to the high-surgical risk, it was decided not to proceed with surgery.
The second patient was 62 years old and only suffered from intermittent claudication. It was decided to proceed with optimal medical therapy and walking rehabilitation, and then reassess the results at 3 months. Intraoperative failures are reported in Table 3.
| Patients | Guidewires | Support catheter | Balloons | Bypass |
|---|---|---|---|---|
| 2 | 1 Terumo®+2 v18® | 3 Trailblazer® | 3 × 60 Savvy® | Yes in the same operative time |
| 16 | 1 Terumo®+2 v18® | 2 Trailblazer® | 5×100 Powerflex® | No |
| 24 | 1 v18® | 2 Trailblazer® | Yes in the same operative time | |
| 25 | 1 v18® | 2 Trailblazer® | No |
Table 3: Endovascular failures.
Postoperative morbidity and mortality
Postoperative complications and re-interventions are reported in Table 4. There was no dissection of the popliteal or tibial arteries secondary to the retrograde puncture and one hematoma at the distal puncture site was observed (4.2%), without the need for surgical revision. There was no 30-day/in-hospital mortality.
| Variables | Patients(N=24) |
|---|---|
| Hematoma at distal puncture site | 1 (4.2) |
| In-stent restenosis | 2 (8.3) |
| Occlusion | 9 (37.5) |
| Endovascular reintervention | 2* (8.3) |
| Open bypass surgery | 2* (8.3) |
| Death | 8** (33.3) |
| *1 plain old balloon angioplasty, 1 drug eluting balloon angioplasty and stenting followed by ilio-deep femoral bypass surgery, 1 femoro-popliteal bypass surgery **Over 26 included patients |
|
Table 4: Post-operative morbidity and mortality.
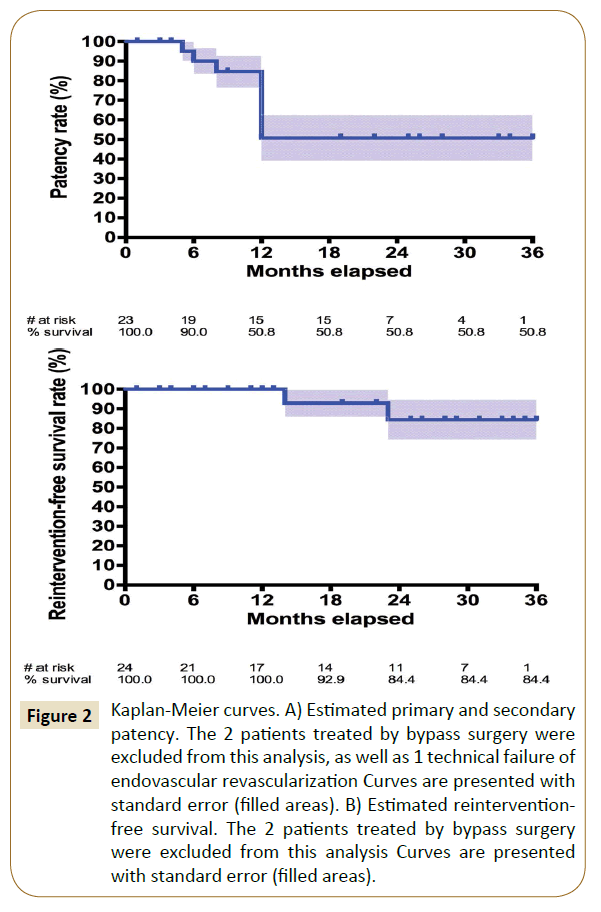
Mean follow-up was 19.5 ± 12.4 months. The two patients who underwent bypass surgery during the initial procedure were excluded from the permeability analysis in order to keep only the permeability of endovascular revascularizations. Estimated primary and secondary patency rates were similar, 90.0% at 6 months and 50.8% at 12, 24 and 36 months respectively (Figure 2A). There were two secondary angioplasties (8.3%) and 2 secondary bypass surgeries (8.3%), leading to an estimated reintervention-free survival of 100.0% at 6 and 12 months and 84.4% at 24 and 36 months (Figure 2B).
Figure 2: Kaplan-Meier curves. A) Estimated primary and secondary patency. The 2 patients treated by bypass surgery were excluded from this analysis, as well as 1 technical failure of endovascular revascularization Curves are presented with standard error (filled areas). B) Estimated reinterventionfree survival. The 2 patients treated by bypass surgery were excluded from this analysis Curves are presented with standard error (filled areas).
Nine patients (37.5% over 24 endovascular treatments) presented with an occlusion of the femoral revascularization during followup. Of the two patients who were not stented due to optimal results of primary angioplasty, the first was Rutherford class 5 and presented with an occlusion at 6 months of his TASC C lesion. It was decided not to perform revision surgery due to the complete healing of the wound in the 15th postoperative day. Afterwards, his general condition deteriorated and did not permit a new revascularization despite the reopening of his wound at 12 months, leading to his death on the 408th post operative day. The second was Rutherford class 3 and presented with an occlusion of his revascularization at 6 months but was asymptomatic at the follow-up visits, probably because of the associated revascularization of the iliac arteries by kissing-stent and the femoral junction by thromboendarterectomy, which allowed the proper development of the deep femoral network. The 7 additional occlusions occurred on stented patients. The first was Rutherford class 3, reocclusion of his TASC D lesion (330 mm) occurred at 6 months and had been treated by 3 Lifestent® (6 mm in diameter, 100 mm long). He did not want a new procedure, despite the persistence of his symptomatology. The second was Rutherford class 5, reocclusion of his TASC D lesion (290 mm) occurred at 6 months and had been treated by 3 Lifestent® (6 mm in diameter, 100 mm long). His wound was completely healed on the 84th postoperative day and therefore he did not need new revascularization. The third occurred at 12 months; the patient was Rutherford class 3 and had received a Lifestent® (6 mm in diameter, 100 mm long) for a TASC B lesion (138 mm). At the follow-up visits, his walking distance was improved and well above 200 m, probably because of the associated revascularization of the iliac artery, which is why no other revascularization was performed. The fourth was a 94 years old Rutherford class 5 patient, reocclusion of her TASC D lesion (160 mm) occurred at 12 months, she had received an Astron Pulsar® (5 mm in diameter, 100 mm long). Her wound was completely healed on the 34th postoperative day but reopened after this, because of severe deterioration of her general condition, no additional revascularization or amputation was proposed. The fifth was Rutherford class 4, re-occlusion of his TASC D lesion (185 mm) occurred at 6 months. It had been treated by 2 Lifestents® (6 mm in diameter, 100 mm long). He was successfully treated by a femoro-popliteal bypass. The 6th was Rutherford class 6, reocclusion of his TASC D lesion (120 mm) occurred at 12 months, despite two endovascular reintervention, including secondary stenting and drug eluting balloon angioplasty, endovascular treatment was insufficient, he was finally treated by ilio-deep femoral bypass surgery. The last one was Rutherford class 3 and his TASC D lesion (190 mm) reoccluded at 12 months. It had been treated by a Complete SE® (6 mm in diameter, 100 mm long) and a SMART Flex® (6 mm in diameter, 200 mm long). He remained completely asymptomatic, which is why no additional revascularization was proposed.
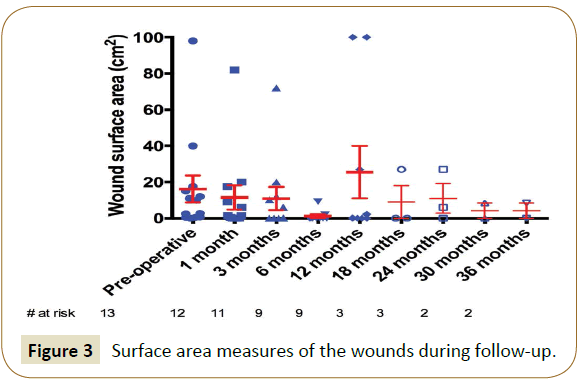
At latest follow-up, 3 patients were suffering from claudication (15.0% over 20 remaining patients, 2 Rutherford class 1 and 1 Rutherford class 3, they were all Rutherford class 3 initially) and 6 patients were in CLI (30.0%, they all remained Rutherford class 5, which was their initial state). Overall, 17 patients (85.0%) were in clinical improvement. Indeed, 7 Rutherford class 3 patients (58.3% of the 12 initial Rutherford class 3 patients) had become asymptomatic. In addition, 4 patients in CLI were completely healed (33.3% of the 12 initial patients). One other patient had completely healed but showed reopening of his wound at 12 months (5.0%). Moreover, 5 patients (25.0%) presented with a decrease in wound surface in a reproducible way over the wound healing center’s consultations. The surface data is shown in Figure 3. In total, 11 patients (55.0%) are now totally asymptomatic and only one major amputation was performed in this series (limb salvage rate of 96.2%) and only one patient required additional minor (toe) amputation (3.8%).
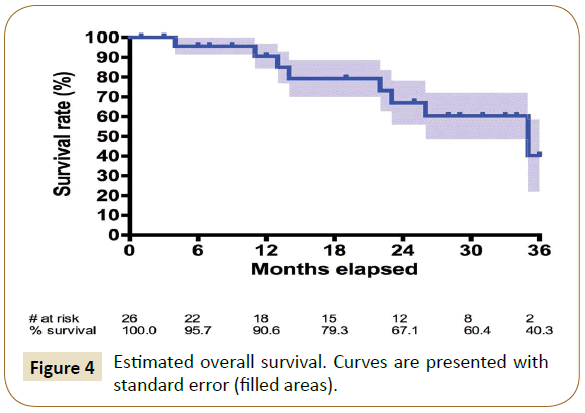
The estimated overall survival rate was 95.7% at 6 months, 90.6% at 12 months, 67.1% at 24 months and 40.3% at 36 months (Figure 4). Eight deaths occurred during follow-up. The first was on the 105th postoperative day in an 81 years old Rutherford class 5 patient. The wound was declining despite the revascularization, and it was chosen in accordance with him and his family, not to perform a major amputation. The second occurred on the 305th postoperative day; patient has already been described above as needing ilio-deep femoral bypass surgery and died of myocardial infarction in the postoperative period. The third occurred on the 637th postoperative day, patient was already described has the only major amputation of the series and died of hemorrhagic shock unrelated to the procedure. The fourth occurred on the 408th postoperative day and has already been described above. Other causes included one septic shock over cholangitis on the 613th postoperative day, 2 cerebral ischemic strokes on the 740th and 988th postoperative days and cardiac decompensation on the 352th postoperative day.
Discussion
Our series technical success of retrograde puncture was comparable to the literature results, ranging between 80 to 90% [11,18,22] By always preparing the patient for the possibility of a retrograde approach, the SAFARI technique was adopted whenever necessary and damage to important collaterals was avoided [13,23]. Technical success of TASC C and D femoropopliteal lesions’ recanalization is close to 90% by antegrade way alone [2,24]. The use of a double approach, anterograde (femoral) and retrograde (popliteal or tibial) helped increase even more this success rate. Recanalization failures were difficult to predict, especially since the calcification index was not calculated on pre-operative scans in this series, and reentry was more difficult to obtain in patients with heavily calcified lesions or diffuse thickening of the media. Hence, the absence of real parietal support was responsible of the 4 failures of retrograde approach, despite an attempted “rendezvous” technique, as the intimal “flap” was impossible to tear. Unfortunately, at this point we did not dispose of a specific reentry catheter, such as the Outback® (Cordis) used in the series of Setacci et al., so we cannot assess if its use would have been beneficial in such cases [25].
All the retrograde punctures were performed with a roadmap technique, with the exception of one case, that was performed with a fluoroscopic guidance alone, since the arteries were heavily calcified and already well visible. However, the experience of ultrasound-guided puncture may be useful to limit radiation exposure and help choose the optimal puncture zone, as demonstrated by Kawadar et al. [18]. The puncture site was usually located at the infra-condylar popliteal level in a supine (knee bent, thigh in abduction) position. The length of the procedure, especially in case of retrograde puncture, implied prolonged standing to limit contrast use, which is often difficult in elderly patients with multiple comorbidities. This is why general anesthesia was systematic. However, it is not essential and some high-risk patients have now been treated under local anesthesia at the femoral access site, with just an adjunctive local anesthesia if a retrograde puncture was needed. In case of pathological popliteal artery, the anterior tibial artery was our second choice and the posterior tibial artery was our last given the difficulty to puncture it. There were no peroneal punctures in this series, however, in our recent experience; peroneal punctures are often performed as a second choice, since it is usually the last remaining run-off vessel in diabetic patients with CLI.
Prolonged balloon angioplasty (180 s) at nominal pressure was applied in an effort to avoid post-angioplasty dissection or elastic recoil [21,26]. All recanalizations received secondary stenting according to the criteria of existing guidelines, with the exception of 2 cases showing optimal results after plain old balloon angioplasty [27]. We did not use drug eluting balloons as a primary strategy in this series, they were only used in case of in-stent restenosis. Nonetheless, they could have been of interest, especially in these 2 cases, since 2 major studies have reported the results of drug coated balloons compared to plain old balloon angioplasty in the SFA, with a primary patency rate of 89.8 % vs. 66.8% at 1 year for the IN.PACT SFA Study, 73.5% vs. 58.8% and 58.6% vs. 53% at 1 and 2 years respectively in the Levant-2 study [28,29]. Moreover, preliminary results also showed lower 12-month restenosis rates using paclitaxel coated balloons before stenting in SFA lesions [30]. The choice of the stent remained at the discretion of the operator, but long selfexpanding nitinol stents were always preferred in order to limit overlap areas. The length of the stented area was defined upon the post-angioplasty angiographic result. Any flow-limiting dissection or residual stenosis ≥30% was stented, regardless of the initial length of the recanalization. This high-rate of secondary stenting was probably related to the length of occlusive lesions and frequency of sub-intimal angioplasty. Systematic stenting remains debaTable, especially in the « no metal left behind » era, and Schillinger et al. reported higher short to mid-term patency rates after plain old balloon angioplasty compared to optional secondary stenting in the treatment of SFA lesions in 2006 [31]. In addition, Chowdhury et al. did not show any significant superiority of primary stenting of the SFA in their long-term meta-analysis in 2014 [32]. In our series, the two non-stented patients presented with early occlusion of their recanalization, which led us today to propose systematic secondary stenting in long re-canalizations. This is supported by the results of the Vienna Absolute study, which was the first randomized study to show superiority of primary stenting over plain old balloon angioplasty for the treatment of moderate-length SFA lesions (63% vs. 37% at 12 months) [31] and the RESILIENT trial (80% vs. 61% at 12 months), which seem to provide sufficient evidence in support of primary stenting for moderate to long-length SFA lesions [33]. Even more so with current-generation nitinol self-expanding stents, showing significant improvement with increased durability and conformability leading to better longterm patency and lower stent fracture rates [34-36].
The primary patency rate of our series was not different from the literature results of CTOs of the SFA, which range from 44 to 92.4% at 12 months [37-41]. Moreover, primary patency was not modified significantly by the use of the SAFARI technique compared to a single anterograde approach, since as shown by Inhat et al. and Dearing et al., it is mainly influenced by the TASC degree and distal run-off [40,42]. We did not use drug eluting platforms in this series, only a bioactive platform in 3 patients with the Heliflex® (Hexacath) stent. However, the Zilver PTX® (Cook Medical) paclitaxel-eluting self-expanding stent has shown significantly higher 1 and 3-year patency rates in the SFA in comparison to both plain old balloon angioplasty and bare metal stenting and could have helped improve our results [43,44]. Another important point to mention is the use of dual antiplatelet therapy after the treatment of long SFA lesions whatever the status of drug eluting platforms. There is no recommendation but most clinical trials prescribe a dual antiplatelet therapy during at least 1 month in the post-operative period, which was the delay respected in a majority of our patients [37-40].
Furthermore, SFA’s patency is not always correlated to the presence of clinical symptoms, it is especially important until wound healing is achieved in CLI patients or collateral arteries are developed in patients suffering from intermittent claudication. This was well stated in our study, since patients presenting with re-occlusions were not always symptomatic nor needed re-intervention. Hence, there was only one major amputation during follow-up, and only two patients could have required an additional major amputation but due to their general condition and in accordance with the patients and their family, it was decided to perform a palliative treatment and avoid an invasive surgery. Otherwise, clinical improvement was observed in 85.0% of the patients at 12 months. It is not easy to compare this rate with the literature results, since most studies only focused on technical success and patency was often the only primary endpoint at short to mid-term follow-up. However, in the series of Hua et al. (38 patients), the limb salvage rate was 80% at 1 year and there were 16% of minor (toe) amputations; in the series of Younes et al. (16 patients), the limb salvage rate was 100% and there were 13% of minor (toe or forefoot) amputations, and in the series of Tay et al. (40 Asian patients), the limb salvage rate was 93% and there were 38% of minor amputations [43-47].
Complications on the retrograde puncture site, such as arteriovenous fistula, hematomas, plaque shift, occlusion, and distal embolization, have been a concern and have limited wider adoption of this technique. In the present study, an infracondylar and caudo-cranial popliteal puncture was chosen over the traditional posterior approach, which kept patients in the supine position. Hence, no arteriovenous fistula complications occurred. There was no dissection of the popliteal or tibial arteries secondary to the retrograde puncture and only one hematoma at the distal puncture site was observed, without the need for surgical revision, reflecting lower morbidity compared to earlier studies [45,48]. This can be explained by a sheathless approach in our study, in contrast with previous authors. This attitude has also been adopted by other authors recently, such as Tokuda et al., who reported significantly less intraoperative and postoperative complications with the sheathless approach compared to the use of a sheath (22.2% in the sheath group [4 or 6 Fr] vs. 2.0% in the support catheter group, p = 0.002) [17]. Moreover, preservation of the P1 or P2 segment of the popliteal artery still permits an above-the-knee bypass in case of secondary occlusion and might also explain the absence of clinical worsening in case of secondary occlusion of the SFA in our series. Finally, since the area of the puncture sites make it difficult to achieve hemostasis by direct compression; we used intraluminal balloon dilatation with mild manual compression at the puncture site as soon as the support catheter was pushed out from the retrograde approach, which might explain the absence of puncture site hemorrhage or false aneurysm in our series. Hence, we believe that the sheathless method should be the first choice for a retrograde popliteal, tibial or even peroneal approach [17].
Limits
There are limitations to the data presented in this series. This was a retrospective observational study, which was performed in only two centers. There was a wide variety of devices used since it was not sponsored by the industry. It has the advantage of demonstrating real life results of the SAFARI technique, whatever the material you have at your disposal. However, the population size was small and there was a potential for bias. This precluded us from doing any subgroup analysis, by lack of sufficient statistical power, especially with regards to the relationship between the devices used and technical success or patency. In addition, the study was initiated in the center of Mont-de-Marsan, which explains the difference in the number of patients included between the two centers.
Arterial Brachial Index (ABI) improvement, often used as a criterion of clinical improvement in clinical studies since it provides a simple, reproducible, and cost-effective assessment of lower-extremity arterial stenosis, was not reported in our study, which constitutes another limitation. However, sensitivity is low, especially in elderly individuals and patients with diabetes, and the values obtained should be interpreted with caution, according to the clinical situation [1,49,50]. Hence, even though it is a simple and useful tool to identify serious stenosis, clinical evaluation and Rutherford staging seemed more appropriate in this setting.
The absence of control group also limits the interpretation of the data presented in this series and the heterogeneity of associated revascularizations lengthened the operative time and skewed the value of postoperative clinical results by artificially improving some of them, especially in case of secondary SFA occlusion. Nevertheless, this series tends to prove that the SAFARI technique is a simple, efficient and reproducible strategy to address long femoro-popliteal occlusions. It can be a useful tool in CLI in the absence of autologous material for the realization of a belowthe- knee femoro-popliteal bypass.
The length of the follow-up was rather short with only a 42-month follow-up for patients included from the beginning of the study and a mean follow-up of 19.5 months, so long-term outcomes could not be evaluated.
Finally, randomized controlled trials comparing clinical efficacy and long-term outcomes after the SAFARI technique against the use of controlled re-entry devices in the management of CTOs with failed anterograde approach have yet to be designed in order to determine the place of re-entry devices in the arsenal of endovascular interventionists, which remains limited by high costs and lack of widespread availability.
Conclusion
The SAFARI technique is a minimally invasive approach, which increases the per-operative technical success rate of femoropopliteal percutaneous angioplasties for TASC C and D lesions, without increasing the rate of local complications or influencing the primary patency rate in the short-run. It helps treat complex occlusive lesions without precluding the use of conventional surgery in a second time and should be considered as a backup option of choice after a failed anterograde recanalization approach.
References
- Norgren L, Hiatt WR, Dormandy JA(2007) Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J VascSurg 45: S5-S67.
- Rabellino M, Zander T, Baldi S (2009) Clinical follow-up in endovascular treatment for TASC C-D lesions in femoro-popliteal segment. Catheter CardiovascInterv 73: 701-705.
- Baril DT, Chaer RA, Rhee RY (2010) Endovascular interventions for TASC II D femoropopliteal lesions. J VascSurg 51: 1406-1412.
- Bolia A, Miles KA, Brennan J (1990) Percutaneous transluminal angioplasty of occlusions of the femoral and popliteal arteries by subintimal dissection. CardiovascInterventRadiol13: 357-363.
- Met R, Van Lienden KP, Koelemay MJW (2008)Subintimal angioplasty for peripheral arterial occlusive disease: a systematic review. CardiovascInterventRadiol31: 687-697.
- London NJ, Srinivasan R, Naylor AR (1994)Subintimal angioplasty of femoropopliteal artery occlusions: the long-term results. Eur J VascSurg 8: 148-155.
- Green JS, Newland C, Fishwick G (1998) Positive outcome following unsuccessful subintimal angioplasty. Eur J VascEndovascSurg16: 266-270.
- Heenan SD, Vinnicombe SJ, Buckenham TM (1994)Percutaneous transluminal angioplasty by a retrograde subintimaltranspopliteal approach. ClinRadiol49: 824-828.
- Shin SH, Baril D, Chaer R (2011) Limitations of the Outback LTD re-entry device in femoropopliteal chronic total occlusions. J VascSurg 53: 1260-1264.
- Smith M, Pappy R, Hennebry TA (2011) Re-entry devices in the treatment of peripheral chronic occlusions. Tex Heart Inst J 38: 392-397.
- Tonnesen KH, Sager P, Karle A (1988) Percutaneous transluminal angioplasty of the superficial femoral artery by retrograde catheterization via the popliteal artery. CardiovascInterventRadiol 11: 127-131.
- Noory E, Rastan A, Schwarzwalder U (2009)Retrogradetranspopliteal recanalization of chronic superficial femoral artery occlusion after failed re-entry during antegrade subintimal angioplasty. J EndovascTher 16: 619-623.
- Spinosa DJ, Leung DA, Harthun NL (2003) Simultaneous antegrade and retrograde access for subintimal recanalization of peripheral arterial occlusion. J VascIntervRadiol 14: 1449-1454.
- Matsi PJ, Manninen HI, Soder HK (1995) Percutaneous transluminal angioplasty in femoral artery occlusions: primary and long-term results in 107 claudicant patients using femoral and popliteal catheterization techniques. ClinRadiol 50: 237-244.
- Zaitoun R, Iyer SS, Lewin RF (1990)Percutaneous popliteal approach for angioplasty of superficial femoral artery occlusions. CathetCardiovascDiagn 21: 154-158.
- Fanelli F, Lucatelli P, Allegritti M (2011)Retrograde popliteal access in the supine patient for recanalization of the superficial femoral artery: initial results. J EndovascTher18: 503-509.
- Tokuda T, Hirano K, Muramatsu T (2014)Asheathless retrograde approach via the popliteal artery is useful and safe for treating chronic total occlusions in the superficial femoral artery. J EndovascTher 21: 289-295.
- Kawarada O, Yokoi Y (2010) Retrograde 3-French popliteal approach in the supine position after failed antegrade angioplasty for chronic superficial femoral artery occlusion. J EndovascTher 17: 255-258.
- Surmely JF, Tsuchikane E, Katoh O (2006) New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique. J Invasive Cardiol 18: 334-338.
- Schmidt A, Bausback Y, Piorkowski M (2012) Retrograde Recanalization Technique for Use After Failed Antegrade Angioplasty in Chronic Femoral Artery Occlusions. J EndovascTher 19: 23-29.
- Zorger N, Manke C, Lenhart M (2002) Peripheral arterial balloon angioplasty: effect of short versus long balloon inflation times on the morphologic results. J VascIntervRadiol 13: 355-359.
- Sangiorgi G, Lauria G, Airoldi F (2012) Retrograde popliteal access as bail-out strategy for challenging occlusions of the superficial femoral artery: a multicenter registry. Catheter CardiovascInterv 79: 1188-1193.
- Gandini R, Pipitone V, Stefanini M (2007) The Safari technique to perform difficult subintimalinfragenicular vessels. CardiovascInterventRadiol30: 469-473.
- Yin M, Jiang M, Huang X (2013) Endovascular interventions for TransAtlanticInterSociety Consensus II C and D femoropopliteal lesions. Chin Med J Engl 126: 415-420.
- Setacci C, Chisci E, de Donato G (2009)Subintimal angioplasty with the aid of a re-entry device for TASC C and D lesions of the SFA. Eur J VascEndovascSurg 38: 76-87.
- Katsanos K, Tepe G, Tsetis D (2014) Standards of Practice for Superficial Femoral and Popliteal Artery Angioplasty and Stenting. CardiovascInterventRadiol 37: 592-603.
- Tsetis D, Belli AM (2004) Guidelines for stenting in infrainguinal arterial disease. CardiovascInterventRadiol 27: 198-203.
- Rosenfield K, Jaff MR, White CJ (2015) Trial of a Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease. N Engl J Med 373: 145-153.
- Tepe G, Laird J, Schneider P (2015) Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 131: 495-502.
- Liistro F, Grotti S, Porto I (2013) Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC CardiovascInterv 6: 1295-1302.
- Schillinger M, Sabeti S, Loewe C (2006) Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 354: 1879-1888.
- Chowdhury MM, McLain AD, Twine CP (2014) Angioplasty versus bare metal stenting for superficial femoral artery lesions. Cochrane Database Syst Rev 6.
- Laird JR, Katzen BT, Scheinert D (2010)Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. CircCardiovascInterv3: 267-276.
- Werner M, Piorkowski M, Thieme M (2013) SUMMIT registry: one-year outcomes after implantation of the EPIC self-expanding nitinol stent in the femoropopliteal segment. J EndovascTher20: 759-766.
- Laird JR, Jain A, Zeller T (2014) Nitinol stent implantation in the superficial femoral artery and proximal popliteal artery: twelve-month results from the complete SE multicenter trial. J EndovascTher21: 202-212.
- Garcia L, Jaff MR, Metzger C (2015) Wire-Interwoven Nitinol Stent Outcome in the Superficial Femoral and Proximal Popliteal Arteries: Twelve-Month Results of the SUPERB Trial. CircCardiovascInterv8.
- Lichtenberg M, Stahlhoff W, Boese D (2013) Superficial femoral artery TASC D Registry: twelve-month effectiveness analysis of the Pulsar-18 SE nitinol stent in patients with critical limb ischemia. J CardiovascSurg Torino 54: 433-439.
- Schoenefeld E, Donas KP, Schönefeld T (2012) Mid-term outcome after endovascular therapy in the superficial femoral and popliteal artery using long stents. VASA Z FürGefässkrankh 41: 49-56.
- Taneja M, Tay KH, Sebastian M (2009) Self-expanding nitinol stents in recanalisation of long-length superficial femoral artery occlusions in patients with critical limb ischaemia. Singapore Med J 50: 1184-1188.
- Grenville JL, Tan KT, Moshonov H (2015) Endovascular first strategy for de novoTransAtlantic Inter-Society Consensus C and D femoro-popliteal disease: mid-term outcomes from a single tertiary referral center. Vascular 23: 31-40.
- Lugmayr HF, Holzer H, Kastner M (2002) Treatment of complex arteriosclerotic lesions with nitinol stents in the superficial femoral and popliteal arteries: a midterm follow-up. Radiology 222: 37-43.
- Ihnat DM, Duong ST, Taylor ZC (2008) Contemporary outcomes after superficial femoral artery angioplasty and stenting: the influence of TASC classification and runoff score. J VascSurg 47: 967-974.
- Dearing DD, Patel KR, Compoginis JM (2009) Primary stenting of the superficial femoral and popliteal artery. J VascSurg 50: 542-547.
- Dake MD, Ansel GM, Jaff MR (2016) Durable Clinical Effectiveness with Paclitaxel-Eluting Stents in the Femoropopliteal Artery: 5-Year Results of the Zilver PTX Randomized Trial. Circulation 133: 1472-1483.
- Hua WR, Yi MQ, Min TL (2013) Popliteal versus tibial retrograde access for subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) technique. Eur J VascEndovascSurg 46: 249-254.
- Younes HK, El-Sayed HF, Davies MG (2015) Retrograde transpopliteal access is safe and effective-it should be added to the vascular surgeon’s portfolio. Ann VascSurg 29: 260-265.
- Tay JS, Ching SS, Tan YK (2016) Endovascular retrograde recanalization in Asian critical limb ischaemia patients. ANZ J Surg.
- Yilmaz S, Sindel T, Luleci E (2005) Ultrasound-guided retrograde popliteal artery catheterization: experience in 174 consecutive patients. J EndovascTher 12: 714-722.
- DachunXunull, Jue Li null, LilingZounull (2010) Sensitivity and specificity of the ankle-brachial index to diagnose peripheral artery disease: a structured review. Vasc Med LondEngl15: 361-369.
- Potier L, Abi Khalil C, Mohammedi K (2011) Use and Utility of Ankle Brachial Index in Patients with Diabetes. Eur J VascEndovascSurg41: 110-116.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences