Arterial Manifestations in Antiphospholipid Antibody Syndrome
Marcos A Marques, Arno von Ristow, Adilson F Paschoa, Andre Luiz Malavazzi, Julio Cesar Peclat de Oliveira and Paula Marques Vivas
DOI10.21767/2573-4482.18.03.5
1Angiology Department, State University of Rio de Janeiro (UERJ), Rio de Janeiro, Brazil
2Vascular and Endovascular Department, Catholic Pontifical University of Rio de Janeiro (PUC-RJ), Rio de Janeiro, Brazil
3Vascular and Endovascular Department, Portuguese Beneficence of Sao Paulo, Sao Paulo, Brazil
4Obstetric Department of the Reference Center of the Woman of the State of Sao Paulo, Sao Paulo, Brazil
5Vascular and Endovascular Department, Universidade Federal do Estado do Rio de Janeiro (UNIRIO), Rio de Janeiro, Brazil
- *Corresponding Author:
- Marcos Areas Marques
Angiology Department, State University of Rio de Janeiro (UERJ)
Boulevard 28 de setembro 77 - Vila Isabel, CEP: 20.551-030, Rio de Janeiro, Brazil.
Tel: +55-21-998590160
E-mail: mareasmarques@gmail.com
Received date: March 14, 2016; Accepted date: April 19, 2016; Published date: April 22, 2016
Citation: Marques MA, Ristow AV, Paschoa AF, Malavazzi AL, Oliveira JCPD, et al. (2018) Arterial Manifestations in Antiphospholipid Antibody Syndrome. J Vasc Endovasc Therapy. 3:5. doi: 10.21767/2573-4482.100075
Abstract
Antiphospholipid antibody syndrome (APS) is classically characterized as venous or arterial thrombosis, recurrent fetal loss and/or thrombocytopenia in patients with antiphospholipid antibodies (anticardiolipin IgM and IgG, lupus anticoagulant and/or anti β2 glycoprotein I IgM and IgG). In spite of deep venous thrombosis of lower limbs being the most common non-obstetric clinical manifestation, there is a clear but less frequent association of APS with the arterial system. This involvement may manifest itself through peripheral arterial disease, acute arterial occlusion and early atherosclerosis. The objective of this review is to clarify some aspects of diagnosis, prevention and treatment that may aid in the clinical management of these patients.
Keywords
Antiphospholipid syndrome; Peripheral arterial disease; Atherosclerosis; Intermittent claudication
Introduction
Antiphospholipid antibody syndrome (APS) is an autoimmune systemic disease characterized by the presence of venous and/ or arterial thrombosis, fetal death and spontaneous repetitive abortion, associated with high titers of antiphospholipid antibodies (APLs) (lupus anticoagulant, IgM anticardiolipin and/ or IgG and/or anti β2 glycoprotein I IgM and/or IgG) [1]. Definitive diagnosis of APS requires the combination of at least one of the clinical criteria with one of the laboratory criteria [2].
Classically, deep venous thrombosis (DVT) and pulmonary embolism (PE) are two of the most common non-obstetric clinical manifestations of APS, affecting up to 38.9% and 14.1% of patients with APS, respectively [3]. In addition, approximately 16.6% of the patients with APS will develop a thrombotic event within the first five years after diagnosis [4].
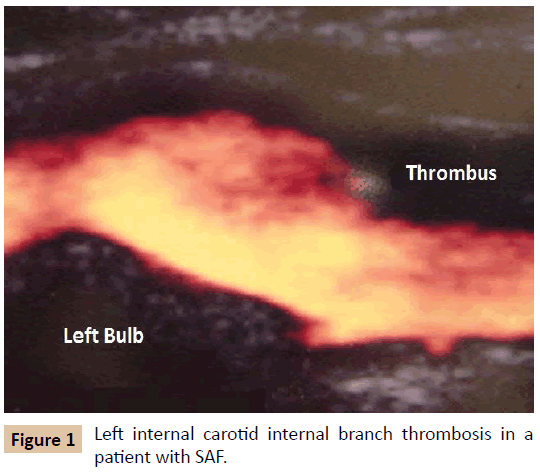
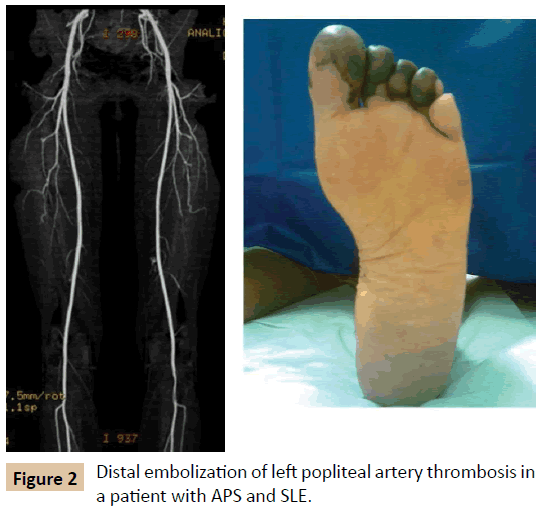
The same study draws attention to the fact that the clinical manifestations associated with the arterial system are also relatively frequent in APS, although less studied, especially with regard to the central nervous system (Figure 1) - ischemic stroke (13.1%), transient ischemic attack (7%) and amaurosis fugax (2.8%) [4]. Less common, but no less striking, is the finding of other acute arterial manifestations, such as arterial thrombosis of the lower limbs (4.3%) (Figure 2) and of the upper limbs (2.7%) [3].
A published meta-analysis associates APS not only with the acute arterial events described above, but also with subclinical cardiovascular manifestations, such as medial-intimal thickening and atherosclerotic carotid disease, ankle-brachial index decrease and renal artery stenosis [4].
These manifestations are secondary to APLs-mediated endothelial cell dysfunction that would bind to endothelial β2 glycoprotein I receptors, which could lead to DVT, early atherosclerotic disease (AD), endothelial cell proliferation, intimal hyperplasia and intimal and medial fibrosis [5].
Another possible form of arterial manifestation of APS, secondary to endothelial dysfunction, is the association with small, medium and large vessel vasculitis, which is characterized when infiltration of inflammatory cells, associated with fibrinoid lesion or necrosis of the vessel wall is present [6]. Vasculitis associated with APLs may be primary or secondary, associated or not with other autoimmune diseases, such as systemic lupus erythematosus (SLE), Takayasu arteritis, giant cell arteritis, Buerger disease and Behçet disease [6].
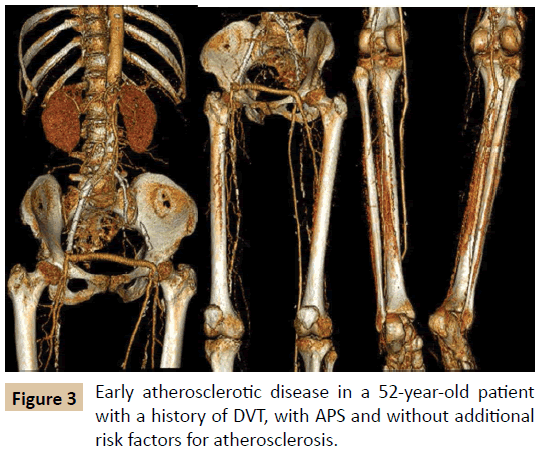
The risk of early atherosclerosis in autoimmune diseases such as APS is known. Although controversial, it may be related to endothelial dysfunction that occurs in chronic systemic inflammatory states and be potentiated by the chronic use of corticosteroids, common in the treatment of APS secondary to SLE [7], or by traditional risk factors such as smoking, diabetes mellitus (DM), systemic arterial hypertension (SAH) and dyslipidemia (Figure 3). The AD in patients with APS draws even more attention because these patients have a different epidemiological profile from the classic atherosclerotic patient, since they are younger, predominantly female and with a low rate of smoking [7].
Evidence Synthesis
Primary thromboprophylaxis
Primary thromboprophylaxis of arterial and venous events remains the central goal in the management of APS. From 0% to 4% of asymptomatic individuals with APLs positivity may develop thrombotic events within a year, especially in those with SLE [8-11]. The control of traditional risk factors, such as SAH, dyslipidemia and obesity, is essential for the prevention of additional damage to the endothelium of these patients [7]. For all patients with triple positive APLs, primary prophylaxis of DVT with heparin is recommended in situations of risk, such as surgery, prolonged immobilization and puerperium [11]. Two meta-analyses suggest that the use of aspirin (ASA) in doses 75-100 mg/day, decreases the risk of the first event of arterial or venous thrombotic in asymptomatic patients with positive APLs, especially in the presence of other risk factors [7,11]. Hydroxychloroquine, an antimalarial drug, may be added to the use of ASA in primary thromboprophylaxis only in those patients with positive SLE and APLs [11].
Secondary thromboprophylaxis
Anticoagulation is the basic medication for secondary thromboprophylaxis in APS [7]. If the initial event is DVT and/or PE, it should be perennial, initially with heparin, either non-fractionated (UFH) or low molecular weight (LMWH) forms followed by warfarin, maintaining INR (International Normalization Rate) between 2 and 3, for the acute treatment and prevention of recurrence.
Arterial thrombotic events have been less well-studied and anticoagulation management is not clearly established [7]. However, there are two basic, non-consensual approaches: a) perennial anticoagulation, initially with UFH or LMWH, and subsequently with warfarin, but with INR greater than 3, or b) or perennial anticoagulation, initially with UFH or LMWH, and subsequently with warfarin, maintaining INR between 2 and 3, associated with antiplatelet therapy [7,11].
The efficacy and safety of direct oral anticoagulants (DOACS) in these patients still remains inconclusive. It should be remembered that the studies that allowed the approval of these medications by the regulatory agencies counted with few cases of APS in proportion to the populations studied. Thus, there is no data available to stimulate the use of DOACS in patients with APS. Nevertheless, the use of DOACS can be considered in those cases of intolerance or allergy to warfarin, or in those in which anticoagulation control is not feasible or recurrent, even with adequate anticoagulant therapy [7].
Fondaparinux, a synthetic, indirect and selective factor Xa inhibitor, may be used in patients with APS who develop a clinical picture of heparin-induced thrombocytopenia [7].
In patients with recurrent arterial or venous thrombosis, in spite of correct anticoagulant therapy, the use of immunomodulator drugs, such as statins and corticosteroids, or even monoclonal antibodies such as rituximab and eculizumab have been employed [11], but still not validated by the main guidelines.
Conclusion
The morbidity and mortality of primary and secondary APS is strongly associated with lesions and endothelial dysfunction mediated by the presence of APLs. The identification of patients with APS with a higher risk of developing venous or arterial thromboembolic events is fundamental for the optimization of primary and secondary prevention.
References
- Marques MA, Murad FF, Ristow AV, Silveira PRM, Pinto JESS, et al. (2010) Acute carotid occlusion and stroke due antiphospholipid antibody syndrome: case report and literature review. Int Angiol 29: 380-384.
- Miyakis S, Lockshin MD, Atsumi T (2006) International consensus statement on an uptodate of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4: 295-306.
- Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, et al. (2002) Antiphospholipid Syndrome. Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis & Rheumatism 46: 1019-1027.
- Ambrosino P, Lupoli R, Di Minno MN (2014) Markers of cardiovascular risk in patients with antiphospholipid syndrome: a meta-analysis of literature studies. Ann Med 46: 693-702.
- Corban MT, Duarte-Garcia A, McBane RD, Matteson EL, Lerman LO, et al. (2017) Antiphospholipid syndrome. Role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. JACC 69: 2317-2330.
- Asherson RA, von Landenberg P (2009) Antiphospholipid antibodies and vasculitis. In: Asherson RA, editor. Antiphospholipid syndrome in systemic autoimmune diseases. Amsterdan 155-184.
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamasthta MA, et al. (2015) The global anti-phospholipid syndrome score in primary APS. Rheumatol 54: 134-138.
- Negrini S, Pappalardo F, Murdaca G, Indiveri F, Puppo F (2016) The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med.
- Cervera R (2015) Morbidity and mortality in the antiphospholipid syndrome during 10-year period: a multicenter prospective study of 1000 patients. Ann Rheum Dis 74: 1011-1018.
- Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I (2011) Evidence-based recommendations for the prevention and long term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at 13th International Congress in antiphospholipid antibodies. Lupus 20: 206-218.
- Ruiz-Irastorza G, Hunt BJ, Khamasthta MA (2007) A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum 57: 1487-1495.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences