Brachio-Cubital Fasciotomy for Resistant Stenosis of Arterio-Venous Fistula
Joon Ho Hong
DOI10.21767/2573-4482.100066
Department of Surgery, Downstate Medical Center, State University of New York, 450 Clarkson Avenue, Brooklyn NY 11203, USA
- *Corresponding Author:
- Joon Ho Hong
Department of Surgery, Downstate Medical Center
State University of New York, 450 Clarkson Avenue
Brooklyn NY 11203, USA.
Tel: 019178862462
E-mail: Joon.hong@downstate.edu
Received date: October 21, 2017; Accepted date: November 01, 2017; Published date: November 06, 2017
Citation: Hong JH (2017) Brachio-Cubital Fasciotomy for Resistant Stenosis of Arterio-Venous Fistula. J Vasc Endovasc Surg. 2:33 doi: 10.21767/2573-4482.100066
Abstract
Purpose: Radio-cephalic arterio-venous fistula can become dysfunctional due to venous outflow stenosis along its course. When the median cubital and the upper arm cephalic vein are small or obliterated, all the forearm cephalic vein blood flow can go through the perforating deep median vein into the brachial vena comitans. These deeply located veins, when dilated, become compressed by the overlying antecubital fascia and bicipital aponeurosis. These ensuing stenoses are usually resistant to endovascular balloon dilatation angioplasty or recur rapidly afterwards. Brachio-cubital fasciotomy was utilized to help relieve the compression and salvage the access.
Methods: For the confirmed resistant stenoses of the AVF, open fasciotomy was performed along the medial aspect of the antecubital fossa. Endovascular angioplasty of the stenotic lesions was accomplished in conjunction with vascular skeletonization while the balloon was inflated inside the AVF.
Results: Nineteen patients recognized to have resistant stenoses of their AVF underwent brachio-cubital fasciotomy. Through the fasciotomy, stenoses were resolved and intra-access pressure reduced to satisfactory levels in all patients. Three patients required resection of the constricted segment and reanastomosis of the mobilized fistula in addition to the fasciotomy. At the follow-up observation of minimum 9 months, all but one of the rescued stenotic lesions stayed patent and required no further intervention.
Conclusions: Radio-cephalic arterio-venous fistula can become dysfunctional due to the development of outflow stenosis from compression by overlying fascia and this stenosis is usually resistant to the dilatation angioplasty. Brachio-cubital fasciotomy resolves these stenoses successfully and allows complete angioplasty with vascular skeletonization and open revision if required.
Keywords
Arterio-venous fistula; Resistant stenosis; Antecubital fascia; Bicipital aponeurosis; Brachio-cubital fasciotomy; Perforating vein; Vena comitans
Introduction
Ever since the distal radio-cephalic arterio-venous fistula (RCAVF) was described by Brescia and Cimino for long-term hemodialysis [1], it remains the preferred vascular access with its well-known durability and minimal complication rate [2]. However, this AVF can become dysfunctional due to the development of venous outflow stenosis along its course [3].
The cephalic vein ascends along the radial aspect of the forearm and gives off the median cubital vein, which crosses the antecubital fossa to join the basilic vein or occasionally the brachial vena comitans in the medial aspects of upper arm. The median cubital vein receives a number of tributaries from the flexor aspect of the forearm and gives off the deep median vein, which perforates the fascial roof of the antecubital fossa to join the venae comitantes of the brachial artery. When the median cubital and the upper arm cephalic vein are small or obliterated, all the forearm cephalic vein blood flow can go into the deeper vena comitans through this perforating deep median vein. The deep median vein and the vena comitans ascend obliquely in close proximity to the brachial artery, and deep under the antecubital fascia and the bicipital aponeurosis [4,5].
With the distal RCAVF creation, the arterialized cephalic vein becomes progressively dilated along with the outflow veins of the median vein and the upper arm cephalic vein. When these outflow veins are small or obliterated, blood flow navigates the antecubital fossa upward through the deep median vein and the vena comitans. These deep veins, when dilated from the access flow, become compressed by the overlying antecubital fascia and the bicipital aponeurosis. The limited vein dilation due to fascial compression in this area leads to a segmental stenosis of the access. The ensuing development of high intra-access pressure causes aneurysmal dilatation with disfigurement and other complications of the access in the forearm.
These stenoses from external compression become resistant to endovascular balloon dilatation angioplasty (EVA) and can recur rapidly afterwards. Extreme pain is felt by most of the patients during balloon inflation at the site of the lesion. A stent placement is not a valid option since the stent may provoke injuries to the adjacent median nerve and brachial artery in a tight space. This paper describes usefulness of brachio-cubital fasciotomy to help relieve the compression and salvage the access.
Methods
Patient selection
Access dysfunction was diagnosed by the history of disproportionate pain upon needle insertion, high venous pressure during dialysis or prolonged bleeding from needle puncture sites after dialysis and confirmed by physical findings of the pulsatile nature of access flow, the presence of thrill or bruit at the site of outflow stenosis, or aneurysmal dilations. Stenosis was defined to be resistant when angioplasty balloon cannot be fully expanded at its bursting pressure; stenosis recoiled immediately or recurrent stenosis developed within 3 month of EVA treatment. Between January 2010 and December 2016, nineteen patients were identified to have resistant stenosis in the cubital area and were treated with brachio-cubital fasciotomy. Intra-access pressure was measured by the height of normal saline solution column in an intravenous extension tube line which was connected to the vascular introducer sheath. Followup examinations were at 3-6 month intervals for a minimum of 9 months up to 6 years or until patient death, kidney transplant, or lost in follow-up. Pertinent patient information was analyzed retrospectively.
Operative procedure
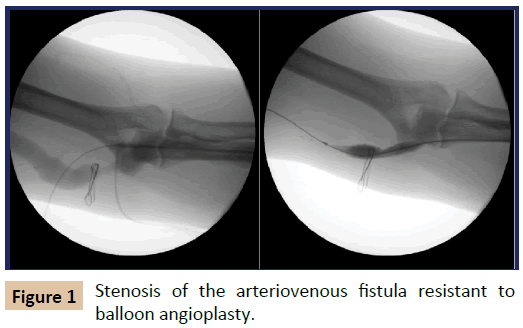
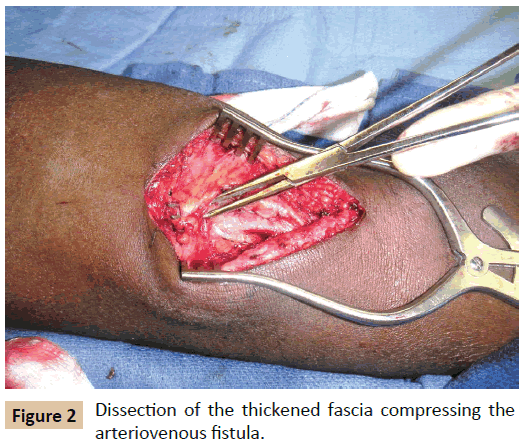
An angiogram was performed to identify the stenotic lesions following the insertion of a vascular introducer sheath into the AVF near the arterial inflow, and EVAs were performed using 8-12 mm balloon catheter (Conquest PTA Balloon Dilatation catheter, CR Bard Peripheral Vascular, Tempe, AZ, USA). When the angioplasty balloon could not be expanded fully over the stenotic lesion on maximum inflation (Figure 1) or the stenosis recoiled immediately upon completion of the angioplasty, open fasciotomy was performed. The AVFs with recurrent stenoses in a short follow-up period of 3 months were also managed by fasciotomy. With the inflated balloon still placed over the stenotic lesion, the location of the stenosis was marked on the skin with the help of fluoroscopic roadmap image. A small longitudinal skin incision was made along the medial aspect of the antecubital fossa. Once the overlying compressive fascia and aponeurosis were exposed, they were dissected from the underlying fistula (Figure 2), and then divided along the course of the fistula using scissors. The fasciotomy was extended when necessary along the upper arm towards the axilla and down to the proximal forearm across the antecubital fossa in order to dissect and divide the side braches off the main conduit vein. Any acute angulation and tugging of the vein was released by this additional procedure, which helped to straighten the compressed vein and render smooth access flow. EVA of the stenotic lesion was completed along with vascular skeletonization while the balloon was inflated inside the fistula. Severely constricted segments beyond EVA capability were resected and AVF was reconstructed by anastomosis of mobilized veins.
Results
Of the 19 patients with resistant stenosis of their AVF, all were African-Americans; 13 patients were men, 6 were women, and the mean age was 61 years. Fourteen AVFs were in the left forearm and 5 in the right forearm. The interval between AVF creation and presentation with hemodynamically significant stenosis ranged from 2 to 93 months with an average of 18 months. At least one, or up to 4 endovascular interventions were performed on the stenotic lesion prior to the fasciotomy. Upon exploration of the stenotic lesion, the overlying fascia and aponeurosis were found to be thick and firm compressing the vein underneath. Through fasciotomy, stenoses were resolved and intra-access pressure reduced in all patients. Three AVFs required resection of constricted segments and end-to-end reanastomosis of the mobilized veins. A stent-graft (10 mm X 4 cm Fluency Plus, CR Bard Peripheral Vascular, Tempe, AZ, USA) was installed in one patient after rupture of the stenotic part following vascular skeletonization and dilatation angioplasty. In 7 AVFs, access flow became excessive upon fasciotomy and was reduced by open plication of the arterialized cephalic vein near the anastomosis to the radial artery.
During the follow-up observation after the fasciotomy, no further intervention was required on the resolved stenotic lesions in the antecubital fossa. One AVF failed in 20 months from severe keloid formation along the needle puncture sites of the access, but the rest of the AVFs were patent and used for dialysis.
Discussion
Once the RCAVF is matured and utilized successfully for longterm dialysis, venous outflow stenosis becomes the most common cause of access dysfunction [6]. Venous stenosis or stricture can develop from an intrinsic pathology or external compression. Intrinsic pathology can be pre-existent such as intimal hyperplasia, or can develop at the site where repeated venipuncture was performed previously. The superficial median vein in the antecubital fossa is the favorite site for venipuncture for intravenous therapy or for blood sampling. In the patients reported herein, the superficial median vein in the antecubital fossa and the cephalic vein in the upper arm were small or obliterated and the access blood flow traversed the antecubital fossa upward through the deep median vein and the vena comitans. In a few other patients, no large superficial median cubital vein was found between the cephalic and basilic veins as is reported in 5-7% of the general population [7,8]. These deep veins develop progressive dilation from the access flow, but the dilation becomes limited by the overlying antecubital fascia and the bicipital aponeurosis, which leads to segmental stenosis over varied periods of time. Although these stenoses were initially managed by a series of EVAs until the limited space under the fascia had no more room for expansion, the extrinsic compression was finally recognized and relieved after a diverse time period. By this time, these stenoses became resistant to EVA or recurred rapidly afterwards. Extreme pain upon balloon inflation was invariably pathognomonic for fascial compression in this area due to the adjacent neurovascular bundle.
Outflow stenosis of the fistula leads to the development of high intra-access pressure and promotes further dilatation of the proximal portion of the fistula. The consequent pulsatile flow may trigger reactive changes in the vessel wall and the adjacent tissue of the overlying fascia, which in turn can cause more compression of the underlying vein, creating a vicious cycle. Repeated EVA can contribute to this cycle. In these patients, the antecubital fascia was found unusually thickened along with the bicipital aponeurosis, a fascial element originating from the biceps tendon and fanning out distally and medially and inserts into the pronator fascia. This aponeurosis, also known as the lacertus fibrosus and the Grace a Dieu fascia, protects the brachial artery from the risk of accidental damage [9]. This aponeurosis may also act as a source of compression on the brachial artery and the median nerve around the elbow and often should be released for a complete volar forearm fasciotomy [10-12].
After the open brachio-cubital fasciotomy relieves the overlying compression, the stenotic lesion is easily dilated by concomitant EVA. The described technique requires only a small skin incision through which the compressing fascia and bicipital aponeurosis can be exposed and divided easily. The main conduit vein can be freed and straightened from any angulation or tugging to render the access flow smooth with minimal turbulence. The strictured segment of the fistula can be resected and reanastomosed. The final outcome of this all-inclusive procedure is gratifying in view of the numerous prior attempts of percutaneous interventions in these patients.
Endovascular stenting can be the next option to overcome the resistant stenosis. However, stent placement was not considered in these patients because of the probable stent-related complications across the bend of the elbow joint and injuries to the adjacent median nerve and brachial artery in a tight space.
For other anatomical sites, surgical decompressions followed by endovascular intervention have been reported to provide the best long-term relief for Paget-Schroetter syndrome [13] and vascular access-associated central vein stenoses [14]. However, unlike the stenoses occurring at the costoclavicular junction, vein stenoses in the cubital area of the arm result not from compression by bony structure, but only from compression by the thickened brachio-cubital fascia in the tight space of the medial brachial fascial compartment.
Angioplasty alone for the communicating stenotic veins to the brachial vein in patients with obliterated superficial veins of the upper arm yielded high initial success but lower patency rates of the matured AVF at 12-21 month follow-up [15]. The communicating veins described in their report were located deep to the antecubital fascia and were actually the perforating deep median vein and brachial venae comitantes according to the latest nomenclature [16]. The addition of fasciotomy to angioplasty and division of side branches could have resulted in better and longerlasting dilation of the communicating vein and straightening of the “N”-shaped configuration.
AVFs in the patients described herein were created in an obtuse angle anastomosis making a loop configuration access and the maturation was augmented by a series of EVAs which helped the access to develop substantial flow rates and vein dilation. With the higher flow rate in the fistula, enlargement of the fistula became intensified in the forearm when the compressed vein in the antecubital area could not be dilated further. This lead to a vicious cycle of making the stenosis relatively worse in the outflow segment, and raising intra-access pressure with progressive dilation of inflow which in turn increased access flow. Therefore, it was necessary to break this cycle by relieving the outflow stenosis and also by reducing excessive inflow in a number of patients.
Outflow stenosis of the AVF should be resolved at all costs to prevent the perils of aneurysmal dilatations with disfigurement, prolonged bleeding, infection at the needle puncture sites, and high recirculation rate leading to ineffective dialysis. Access stenosis from external compression by the thickened fascia is best resolved by open surgical fasciotomy and additional necessary revisions to achieve long-term patency.
Conclusion
The brachio-cubital fasciotomy effectively resolves the resistant stenosis of the arterio-venous fistula by relieving vein compression in the elbow area. This technique also allows an extended fasciotomy along the course of the fistula, vascular skeletonization for completion angioplasty and open revision if required.
A preliminary study was presented at the Vascular Access for Hemodialysis XIII Symposium of Vascular Access Society of America, 5/2012, Orlando FL –USA.
References
- Brescia MJ, Cimino JE, Appel K, Hurwich BJ (1966) Chronic hemodialysis using venipuncture and a surgically created arterio-venous fistula. N Engl J Med 275: 1089-1092.
- NKF-DOQI Clinical Practice Guidelines for Vascular Access (2006) Amer J Kidney Disease 48: S177-S247.
- Mousa AY, Dearing DD, Aburahma AF (2013) Radiocephalic fistula: review and update. Ann Vasc Surg 27: 370-378.
- Shenoy S (2009) Surgical anatomy of upper arm: what is needed for AVF planning. J Vasc Access 10: 223-232.
- Ellis H, Feldman S, Harrup-Griffiths W (2010) The clinical anatomy of the antecubital fossa. Br J Hosp Med 71: M4-M5.
- Pirozzi N, Garcia-Medina J, Hanoy M (2014) Stenosis complicating vascular access for hemodialysis: indications for treatment. J Vasc Access 15: 76-82.
- Mikuni Y, Chiba S, Tonosaki Y (2013) Topographical anatomy of superficial veins, cutaneous nerves, and arteries at venipuncture sites in the cubital fossa. Anat Sci Int 88: 46-57.
- Vucinic N, Eric M, Macanovic M (2016) Patterns of superficial veins of the middle upper extremity in Caucasian population. J Vasc Access 17: 87-92.
- Snoeck O, Lefevre P, Van Sint Jan S (2014) The lacertus fibrosus of the biceps brachii muscle: an anatomical study. Surg Radiol Ana 36: 713-719.
- Cevirme D, Aksoy E, Adademir T, Sunar H (2015) A perplexing presentation of entrapment of the brachial artery. Case Rep Vas Med 2015: 1-3.
- Braun RM, Spinner RJ (1991) Spontaneous bilateral median nerve compressions in the distal arm. J Hand Surgery 16: 244-247.
- Kumar H, Das S, Gaur S (2007) Entrapment of the median nerve and the brachial artery by the lacertus fibrosus. Arch Med Sci 3: 284-286.
- Urschel HC, Rassuk MA (2000) Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg 69: 1663-1668.
- Illig KA (2011) Management of central vein stenoses and occlusions: the critical importance of the costoclavicular junction. Semin Vasc Surg 24: 113-118.
- Kim EY, Won JH, Kim JD, Lee SH, Oh CK (2014) Angioplasty of communicating veins to the brachial vein in hemodialysis patients with obliterated superficial veins of the upper arm. Clini Radiol 69: 703-708.
- Black CM (2014) Anatomy and Physiology of the Lower-Extremity Deep and Superficial Veins. Tech Vasc Inter Radiol 17: 68-73.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences