Cardiac Pacing and Transcatheter Aortic Valve Implantation (TAVI): A Literature Update
Antonio da Silva Menezes Junior, Pedro Paulo Clark de Oliveira, Larissa Favoretto Almeida and Cristiano de Souza Soares
DOI10.21767/2573-4482.18.03.11
Antonio da Silva Menezes Junior*, Pedro Paulo Clark de Oliveira, Larissa Favoretto Almeida and Cristiano de Souza Soares
Pontifical Catholic University of Goiás, Medical, Pharmaceutical and Biomedical School, Goiânia -Goiás Brazil
- *Corresponding Author:
- Antonio da Silva Menezes Junior
Pontifical Catholic University of Goiás
Medical, Pharmaceutical and Biomedical School
Goiânia, Goiás, Brazil
Tel: 6232245813
E-mail: a.menezes.junior@uol.com.br
Received Date: June 10, 2018; Accepted Date: July 10, 2018; Published Date: July 20, 2018
Citation: Junior ADSM, de Oliveira PPC, Almeida LF, et al. (2018) Cardiac Pacing and Transcatheter Aortic Valve Implantation (TAVI): A Literature Update. 3:11.
Abstract
Aortic valve replacement is a routine procedure with acceptable risk. In some cases, mortality is high, contraindicating the procedure. The minimally invasive transcatheter aortic valve implantation seems to be an alternative, reducing morbidity and mortality. In this procedure, a bioprosthetic valve is introduced through a catheter and fixed within the injured native aortic valve. Even though the technique is considered comparatively safe, the risks of complications exist and they that have been established by individuals after the technique, for example new-onset permanent left bundle branch block (LBBB) and the need for permanent cardiac pacing implantation. A systematic review of literature of PUBMED was carried out using “10 years” and “free full texts” as filters, containing the terms “TAVI,” “pacing,” and “complications,” finding a total of eight articles. Other databases, such as SCIELO, Google Scholar and MEDLINE were used to give background, consistency and profundity to the text. The implantation of aortic valve prosthesis per catheter seems to be a valid modality for high–surgical risk patients with aortic stenosis. The results of this series of patients suggest that the need for a definitive pacemaker after endovascular treatment is not inexorable and is not easily predicted by the risk factors described so far.
Keywords
Transcatheter aortic valve implantation (TAVI); Aortic valve stenosis; Cardiac pacing; Complications
Aortic Stenosis (AS) is one of the most common valvular heart diseases in the world. It affects 2% of people over the age of 65, 3% over 75, and 4% over 85 years old, and the incidence seems to increase with the aging of the population [1]. It is an insidious disease with a long latency period, known by fast development after the beginning of symptoms, resulting in a high percentage of death among untreated patients [2].
Transcatheter aortic-valve implantation (TAVI) is a technique in which a bioprosthetic valve is introduced through a catheter and fixed within the injured natural aortic valve [2]. It is a different to conservative operation for patients with severe aortic stenosis at raised operating risk. It also raises the survival and quality of patients’ life [3]. Nowadays, despite this new technology, the surgical valve replacement is the treatment of choice for symptomatic severe aortic stenosis [4].
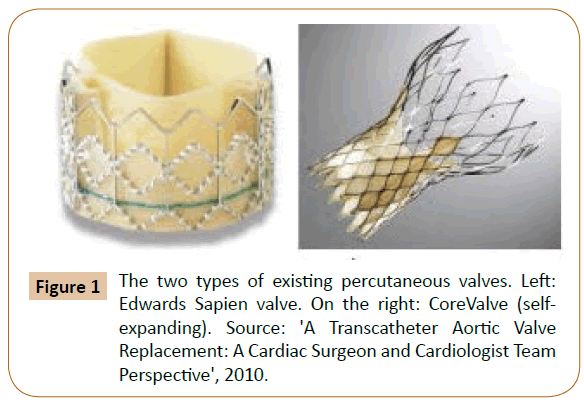
More than a few valves models can be implanted percutaneously in aortic level; however, the greatest data has been increased using two of them, the “Edwards SAPIEN prosthetic heart valve” and the “CoreValve ReValving System” [5]. The first, Edwards SAPIEN, involves a balloon-inflatable, cylindrical border composed of stainless steel, to which is involved a trifoliate, biological equine pericardium heart valve, then, a material skirt is stitched to the border and purposes to moderate paravalvular aortic regurgitation.
This valve is presently presented in two sizes. The attaching of the prosthesis and function of the valve are both intra-annular [5]. The second one is CoreValve ReValving System, self-expandable, trilevel frame composed of nitinol, trifoliate and a porcine pericardium heart valve (Figure 1) [5].
Aortic stenosis frequently affects individuals in the high age group, with a consequent high rate of comorbidities. It is estimated that approximately one-third of patients with characteristic aortic stenosis are deferred from the surgical treatment because of the high risk of postoperative mortality [4].
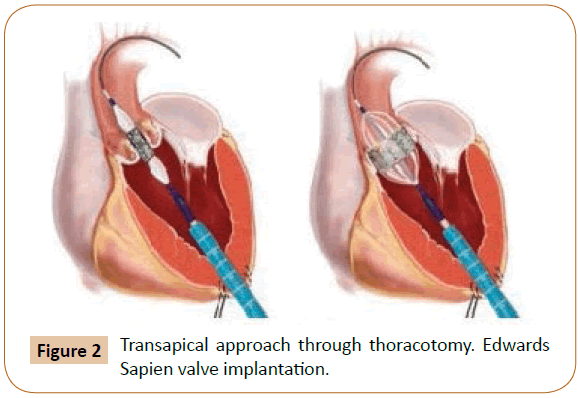
TAVI has become “the standard cares for high-surgical risk or inoperable patients with severe aortic stenosis” [1]. It enables percutaneous implantation of a novel aortic valve with the common of procedures achieved via the transfemoral way under local anesthetic with transapical, transaortic, and subclavian artery access as an alternative, dependent on patient vascular anatomy (Figure 2) [1].
The area near the valve is covered by the endoprosthesis, which compresses the valve annulus and nearby structures, including constituents of the components of the cardiac conduction system. The atrioventricular node and the left branch of the bundle travel inside the fibrous body, adjacent to the non-coronary cusp of the aortic valve and may be affected by the device [6].
Recent clinical studies have demonstrated the feasibility, safety, and efficacy of this type of intervention with quite encouraging results, albeit in the short and medium term [7].
Atrioventricular block (AVB) of the cardiac conduction system and the need for permanent pacemaker implantation (PPI) are frequent complications after aortic valve replacement, whether surgical or percutaneous [3].
Methods
Eligibility criteria
A systematic review of literature of PUBMED was carried out using “10 years” and “free full texts” as filters, containing the keywords “TAVI”, “TAVI AND Pacing” and “TAVI AND Complications” to identify the eligible articles. From this search, a total of 10 articles were selected. Other databases, such as SCIELO, Google Scholar and MEDLINE were used to give background, consistency and depth to the text. There were no language restrictions.
Inclusion and exclusion criteria
Articles were excluded if they were not related to the complications involved in people after the placement of the transcatheter aortic valve implant as well as articles in which the focus and aggravation of the changes were diseases that were already pre-established or that another possible pre-existing genetic cause had been poisoned.
Published cases of acute events related to the implantation of TAVI, for which the information was available were included.
The research, because it presents only free articles, may contain publication biases, for which it is necessary that other studies involving this data be performed and compared with those presented here.
Results
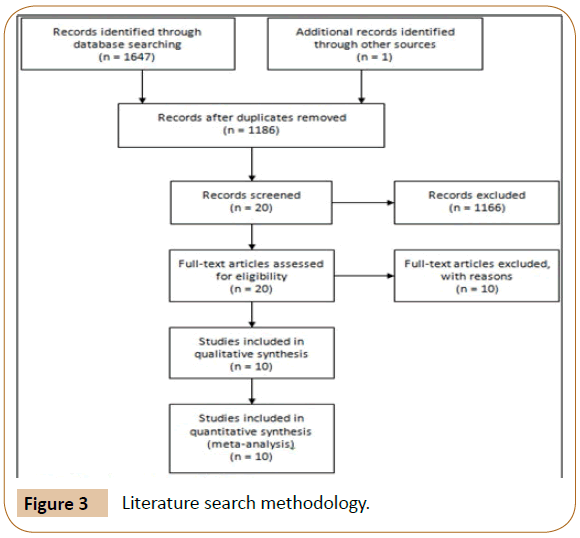
The literature search initially identified 1647 relevant titles from PUBMED, SCIELO, Google Scholar and MEDLINE. 462 articles were deleted because of duplicate data. Finally, after reading the titles and abstracts, 20 articles were selected for complete reading and 10 were used in the meta-analysis. The study selection process and reasons for exclusion are summarized in Figure 3.
Based on the analysis of the articles, it was possible to predict the most frequent complications after TAVI placement. Studies have investigated the possible association between transcatheter aortic valve implantation and reduction of coronary flow, which would lead to myocardial lesion reflected by post-procedural increase in serum troponin I [8]. However, there was no positive correlation with this finding, the existence of a decrease in blood pressure recovery time only being evident compared to the duration of rapid pacing [8].
Another complication was found in relation to the appearance of a new-onset and permanent left bundle branch block after TAVI, often requiring implantation of a permanent pacemaker (PPM), which, however, did not increase the mortality rate of the patients [9-12]. The studies point out the need for careful and longer monitoring for PPM indication during follow-up and to evaluate the effect of persistent left bundle branch block on recent initiation [9,10,12].
Among the criteria used to place PPM, based on the scientific literature, we can cite [4,5,10,11]: a) complete AV block of recent onset; b) new Mobitz Type II AV lock; c) new left bundle branch block with prolongation of the PR interval; d) new left bundle branch block with atrial fibrillation with slow ventricular response and e) temporary asystole during the procedure.
The clear majority of patients requiring PPM placement are elderly (81%) and males (59.3%), these being these the ones who spend the most time in hospital because of the complications of transcatheter valve implantation aortic disease [3,4]. Hospital stay time may be increased if there is a previous “presence of right bundle branch block (RBBB), use of CoreValve prosthesis and basal transaortic gradient >50 mmHg” [3]. Among the patients who had any of the factors mentioned above, the probability of needing PPM was 63%, compared to 4% in patients in whom none of these predictors were present [3].
Ventricular atrial (AV) block has been revealed to be another conduction abnormality generated by TAVI placement and may be related to “higher incidences of mortality, sudden cardiac death, and left ventricular dysfunction” [11,4]. In a study developed in 2015, it was determined that high-grade AV block was developed in 18% of the patients analyzed in 1.2 ± 1.1 days after TAVI, with 29% being submitted to the implant of PPM in 2.2 ± 2.1 days [11].
In a single study found in the database searched, it was possible to notice the development of a severe aortic insufficiency of the valve prosthesis 16 months after TAVI [4]. In order to solve the problem, CoreValve Evolut R prosthesis was implanted just above the LV-Edge of the JenaValve that was used. The treatment option is valid for a transvalvar flaw made explicit in the case [4].
For a better understanding of what was exposed in the review, a table was elaborated with the main articles used and their most relevant points, such as their titles, year of publication, authors, journal and a preview of the conclusion and main points of the results (Table 1).
| SOURCE | DESIGN | YEAR | MAIN RESULTS | CONCLUSION |
|---|---|---|---|---|
| KAHLERT et al. [8] | Clinical trial | 2015 | There were no significant correlations between the dynamics of the coronary flow, VCFR and area under the TnI curve (AUC) over 72 hours, between the amount of HITS and the TnI AUC, in patients with transfemoral TAVI. The positive relationship between the duration of rapid pacing and the subsequent recovery time of blood pressure and the AUC of TnI was seen. | Myocardial injury after TAVI appears to be related more to hypoperfusion-induced ischaemia than to periprocedural microembolisation. |
| ANDO et al. [9] | Meta-analysis | 2016 | In 4049 patients perioperative and long-term mortality were compared and patients with NOB-BAEP presented a higher rate of definitive pacemaker implantation during follow-up in the medium term. | New‐onset persistent left bundle branch block after the placement of TAVI has association with an increased rate of PPI however it did not negatively affect cardiovascular mortality. |
| URENA et al. [10] | Clinical trial | 2015 | The rate of LBBB after TAVI and permanent pacemaker implantation rate (MPC) are, respectively, about 27% and 17%. The incidence of new implants of BCRE and PPM is higher after using the CoreValve CoreValve self-expanding system (Medtronic Inc., Minneapolis, MN, USA) compared to the Edwards SAPIEN / SAPIEN XT valve (Edwards Lifesciences LLC, Irvine, CA , USA) expandable by balloon. Therefore, the increased risk of the need for PPM associated with the CoreValve prosthesis compared to the SAPIEN / SAPIEN XT valve from Edwards was confirmed. A slow reduction was observed in the rate of conduction abnormalities and the requirement of PPM associated with both types of transcatheter valves over time. | Due the high frequency of complications associated with TAVI implantation, such as conduction disorders and the need for PPM, there is great concern about its use. However, the use of a balloon expandable valve at a more aortic implantation site can reduce these complications. |
| HAMDAN et al. [11] | Clinical trial | 2015 | Analyzes showed the length of the MS as the independent predictor of the most powerful pre-procedure of high-grade AV block, the difference between the length of the MS and the depth of implantation as the most powerful independent predictor of high-grade AV block, while the difference between MS length and depth of implantation and calcification in the basal septum were the most powerful independent predictors of MPP implantation. | Short MS, insufficient difference between MS length and implantation depth, and the presence of calcification in the basal septum, may occur after the placement of TAVI with self-expandable valves. |
| AKIN et al. [12] | Clinical trial | 2012 | All patients were successful with TAVI. Baseline ECG and intracardiac EC showed higher QP, longer HA and HV interval in patients requiring pacemakers compared to the control group. Multivariate analysis revealed that only the new LBBB, QRS duration> 120 msec and a PQ interval> 200 milliseconds immediately (within 60 minutes) after aortic valve implantation were high-grade (Grade II and III) degree) | Cardiac disturbs, especially of conduction, are common after the placement of TAVI. Sometimes, the need of the pacemaker is inevitable. |
| PIAZZA et al. [5] | Review article | 2008 | After identification of the origin of the coronary arteries and the location of the left branch | Anatomic knowledge of the aortic valve is useful in the interpretation of the principles of its percutaneous replacement. |
| in relation to the positioning of the prosthesis minimizes the risks of coronary ischemia and abnormalities conduction that may occur during the valve implant. The aid of echocardiography, angiography or multislice and computed tomography may reduce the possibility of patient-prosthesis maladjustment. | ||||
| P.A. et al. [4] | Clinical case study | 2010 | 8 patients treated with aortic stenosis treated with TAVI. All patients had at least one predictive characteristic of high-grade atrioventricular block after the procedure. One patient had an in-hospital death due to tamponade and the other the intervention was successful. During the clinical follow-up of 4 to 12 months, there were no deaths or new atrioventricular block of 2nd or 3rd grades, with zero rate of definitive pacemaker. | TAVI is an alternative for high-risk surgical patients with aortic stenosis. The use of a definitive pacemaker is debatable and predictable |
| MONTEIRO et al. [3] | Clinical trial | 2017 | At 30 days after TAVI, 20.1% of the patients needed IPP. These patients were approximately 82 years old and mainly male. It was observed that the time of hospital stay was higher in those submitted to IPP, however the IPP had no relation with all causes of deaths, nor deaths with cardiovascular etiology. CoreValve® prosthesis and basal transaortic gradient> 50 mm Hg were predictors of IPP. | BRD, mean aortic gradient> 50 mmHg and CoreValve® are independent predictors of post-TAVI MPD implantation. MPD implantation occurred in approximately 20% of TAVI cases, which prolonged hospitalization, but did not affect mortality. |
| LEON et al. [2] | Clinical trial | 2010 | In one year, all-cause mortality was lower with TAVI (30.7%) compared to standard therapy (50.7%), the rate of cardiac symptoms (Class III or IV of the New York Heart Association) was lower in those with TAVI than in those who received standard therapy and there was no deterioration in the functioning of the biological prosthesis. At 30 days, TAVI was related to a higher incidence of stroke and major vascular complications compared to standard therapy. And the rate of death from any cause or recurrence of hospitalization was 42.5% with TAVI, compared to 71.6% with standard therapy. | TAVI, compared with standard therapy, reduced rates of death from any cause in patients with severe aortic stenosis who were not patients suitable for surgery. |
| BAJRANGEE et al. [1] | Clinical trial | 2017 | In 147 patients, predominantly males and mean age of 82 years submitted to TAVI the survival rate in thirty days, one year and two years were respectively 90.5%, 83% and 71%. The greatest predictors of mortality in the first month were renal failure and major vascular complications. | Through this review it was possible to verify favorable rates of survival 30 days, 1 year and 2 years, after setting TAVI. There was procedural success and complication rates were similar to those reported internationally. |
Table 1: Study characteristics.
Discussion
According to Akin et al. considering the risk factors for complete AV block after surgical valve replacement, “the previous aortic regurgitation, pulmonary hypertension, myocardial infarction, and postoperative electrolyte imbalance are the main risk factors. The strongest predictor of pacemaker requirement is the right bundle branch block (RBBB) on surface ECG” [12]. In TAVI, the total of local impairment is influenced by factors such as calcification at the surgical site, height of the site of implantation in the left ventricular outflow tract, intensity of the trauma that occurred during the procedure (balloon valvuloplasty, balloon to aortic annulus relation and post-TAVI dilatation) and aortic annulus geometry [12].
The definitive pacemaker implant is needed in approximately 3% to 8% of cases after surgical treatment [4]. Distinct surgical valve replacement, where the degenerated valve tissue is excised, catheter treatment does not exclude the stenotic valve, with an inevitable compression of the valve annulus and adjacent structures, especially the atrioventricular node and its left branch, which are adjacent to the non-coronary cusp of the aortic valve, within the central fibrous tendon. Such mechanical compression could explain the difference in pacemaker requirement between these two therapeutic modalities [4].
PPI has been related to the compromised left ventricular systolic function. This compromise is supposed to be “secondary to the negative impact of PPI on LVEF” produced by a dyssynchrony led by “artificial electromechanical activation in left ventricular performance” [3].
The native aortic valve is located next to the AV node and to the His bundle. Consequently, TAVI might cause injury to the infra- Hisian conduction system and may occur “due to direct pressure [6] and compression, hemorrhage/hematoma, ischemia or inflammation of the His bundle and compact AV node during the placing or expansion of the prosthesis” [3].
After de procedure, TAVI has been shown to improve left ventricular systolic function [3]. However, some studies have demonstrated that hemorrhagic and renal disorders may occur after TAVI. In the Leon et al. study, larger hemorrhagic individuals were in the TAVI group than were in the standard therapy group [2], whereas in the Bajrangee et al. study the bleeding rate was considerable, reaching 27.7%, with the need for red blood infusion in 25.7% of patients. After investigation, they considered that a great part of the hemorrhagic disturbances were associated with bleeds of the gastrointestinal tract, possibly related to the anticoagulation. However, it does not rule out the possibility of a hemorrhagic disorder and the subsequent transfusion of red blood cells after TAVI [1].
Regarding renal disorders, Bajrangee et al. found that 69% of patients had acute kidney injury and 60% had chronic renal insufficiency at the beginning of the study. Therefore, 9% had no renal impairment and had renal disorders after TAVI. This raises the question of the safety of this procedure for patients with similar conditions and the need for follow-up of patients with TAVI post-procedure [1].
In the study by Leon et al. one of the complications associated with TAVI was frequent paravalvular regurgitation [2]. Such an episode was also recorded by Bajrangee et al. as a significant complication and may cause higher mortality rates in the long term [1].
Spills remain a major complication after TAVI. It has recently been raised that there are new perfusion deficits in patients following TAVI, probably due to the appearance of emboli. Phillip et al. reported that new outbreaks of cerebral diffusion were detected in 84% of patients after TAVI, being more frequent than after conventional heart surgery. The pattern of lesions observed in this study (multiple small lesions scattered in both hemispheres) contributes to the hypothesis raised earlier on the appearance of emboli [8]. Thus, it was found that strokes occur more frequently in patients undergoing TAVI than those who received standard therapy. However, at one year, the death rate from any cause was lower with TAVI than with standard therapy [2].
In contrast, even with the possible complications, most of the studies showed that the death rate after TAVI is inferior to that after the standard therapies, greatly reducing the rate of death from any cause [1,2]. “In the first year, only five patients” in the Leon et al. study required to be treated with TAVI to “avoid one death, and only three patients had to be treated to prevent death or repeat hospitalization” [2].
Therefore, the advantages and disadvantages of TAVI are still controversial, and there is no homogeneity between studies on the dual risk/benefit relationship. Thus, there is a need to do continuous patient follow-up with TAVI to diagnose possible complications and to treat possible conduction disturbances.
Conclusion
The implantation of aortic valve prosthesis through catheter seems to be a valid modality for high surgical risk patients with aortic stenosis. The results of this series of patients suggest that the need for a definitive pacemaker after endovascular treatment is not inexorable and is not easily predicted by the risk factors described so far. Besides, the most frequent post-TAVI complication is the permanent cardiac pacing implantation.
TAVI is the procedure of choice considered feasible and safe. Since the site of implantation of the valve prosthesis is close to septal cardiac structures with important function, conduction disorders are frequent, requiring cautious surveillance for at least seven days after the procedure.t was also possible to conclude that the need for PPM after endovascular treatment is not easily predicted by risk factors, since the studies emphasize the need for a careful and longer follow-up of PPM indication during follow-up [9-14].
References
- Bajrangee A, Coughlan JJ, Teehan S, O Connor C, Murphy RT, et al. (2017) Early and mid-term outcomes after transcatheter aortic valve implantation (TAVI) in Ireland. IJC Hear Vasc 16: 1-3.
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, et al. (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363: 1597-1607.
- Monteiro C, Ferrari ADL, Caramori PRA, Carvalho LAF, Siqueira DA de A, et al. (2017) Permanent pacing after transcatheter aortic valve implantation: Incidence, predictors and evolution of left ventricular function. Arq Bras Cardiol pp: 550-559.
- Lemos PA, Mariani J, Esteves Filho A, Kajita LJ, Cardosos LF, et al. (2010) Transcatheter aortic valve implantation without permanent pacemaker in a series of consecutive cases: Is it possible to predict the risk of atrioventricular block? Rev Bras Cardiol Invasiva 18: 135-139.
- Piazza N, de Jaegere P, Schultz C, Becker AE, Serruys PW, et al. (2008) Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Cardiovasc Interven 1: 74-81.
- Sarmento-Leite R, De Quadros AS, Prates PRL, Voltolini I, Conti E, et al. (2009) Marca-passo permanente após implante percutâneo valvular aórtico: A necessidade é maior que imaginávamos? Rev Bras Cardiol Invasiva 17: 476-483.
- Perin MA, Sandoli de Brito F, Oliveira Almeida B, Pereira MAM (2009) Percutaneous aortic valve replacement for the treatment of aortic stenosis: Early experience in Brazil. Arquivos Brasileiros de Cardiologia 93: 299-306.
- Kahlert P, Al-Rashid F, Plicht B, Wild C, Westholter D, et al. (2016) Myocardial injury during transfemoral transcatheter aortic valve implantation: An intracoronary Doppler and cardiac magnetic resonance imaging study. Euro Interv 11: 1401-1408.
- Tomo Ando M, Hisato Takagi P (2016) The prognostic impact of new-onset persistent left bundle branch block following transcatheter aortic valve implantation: A meta-analysis. Clin Cardiol 9: 544-550.
- Marina Urena M, Josep Rodés-Cabau M (2015) Managing heart block after transcatheter aortic valve implantation: From monitoring to device selection and pacemaker indications. Euro Interv 11: W101-W105.
- Hamdan A, Guetta V, Klempfner R, Konen E, Raanani E, et al. (2015) Inverse relationship between membranous septal length and the risk of atrioventricular block in patients undergoing transcatheter aortic valve implantation. JACC 8: 218-228.
- Akin I, Kische S, Paranskaya L, Schneider H, Rehders TC, et al. (2012) Predictive factors for pacemaker requirement after transcatheter aortic valve implantation. Cardiovasc Disord 12: 87.
- Ruel M, Labinaz M (2010) Transcatheter aortic valve replacement: a cardiac surgeon and cardiologist team perspective. Current Opinion in Cardiology. 25: 107-113.
- Serruys PW (2010) Transcatheter aortic valve implantation, tips and tricks to avoid failure. Informa Healthcare pp:113-114.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences