Novel Animal Model of Large Vessel Venous Thromboembolism to Assess the Effectiveness of Thrombus Removal Therapies
Neal B. Shah, Riyaz Bashir, Brian G. Firth, Marvin L. Woodall, Michael J. Cerminaro, Nicholas Green, Grzegorz L. Kaluza, Gerard Conditt and Geng Hua Yi
Published Date: 2019-06-15DOI10.21767/2573-4482.19.04.14
Neal B. Shah1, Riyaz Bashir2*, Brian G. Firth3, MarvinL. Woodall3, Michael J.Cerminaro3, Nicholas Green3, Grzegorz L. Kaluza4, Gerard Conditt4 and Geng Hua Yi4
1Department of Internal Medicine, Temple University Hospital, Philadelphia, PA, USA
2Department of Cardiovascular Diseases, Temple University Hospital, Philadelphia, PA, USA
3Thrombolex Inc., New Britain, PA, USA
4Skirball Center for Innovation-Cardiovascular Research Foundation, New York, NY USA
- *Corresponding Author:
- Bashir R
Department of Cardiovascular Diseases Temple University Hospital, Philadelphia, PA USA
Tel: +215 316 0232
E-mail: riyaz.bashir@tuhs.temple.edu
Received date: February 28, 2019; Accepted date: June 08, 2019; Published date: June 15, 2019
Citation: Shah NB, Bashir R, Firth BG, Woodall ML, Cerminaro MJ, et al. Novel Animal Model of Large Vessel Venous Thromboembolism to Assess the Effectiveness of Thrombus Removal Therapies. J Vasc Endovasc Therapy 2019, Vol.4 No.2:14.
Abstract
Background: Venous thromboembolic disease, including deep vein thrombosis and pulmonary embolism, accounts for nearly 180,000 deaths annually in the United States. It is the third most common cause of cardiovascular morbidity and mortality, and is usually caused by a thromboembolism of a large vessel like the pulmonary artery or ilio-caval vein.
Objectives: In order to develop more effective treatments for the thromboembolic disease in these large vessels, animal models are necessary. Previous research has been primarily focused on using murine models, which has aided in developing novel pharmacological therapies; however, given the limited size of the large vessels in these models, they are not representative of the clinical presentation in humans.
Methods: A venous blood sample from a Yucatan pig was coagulated in in-vitro and subsequently introduced via the femoral vein. Complete occlusion and cessation of flow across the posterior vena cava was established. A novel infusion catheter-Bashir™ Endovascular Catheter-was advanced into the thrombotic vena cava. The infusion basket was expanded, thrombolysis was initiated, and results were documented by sequential venograms. Thereafter, the catheter was positioned in the pulmonary artery to evaluate pulmonary artery pressures and mixed venous oxygen saturations.
Results and Conclusion: We successfully developed a novel animal model in which we cause a large vessel occlusion by introducing a thrombus into the posterior vena cava. Subsequently, we restored the venous flow by percutaneously dissolving the thrombus using a novel infusion catheter. We believe this model will aid in the development and evaluation of future thrombus reduction therapies
https://maviyolculuk.online/
https://mavitur.online/
https://marmaristeknekirala.com.tr
https://tekneturumarmaris.com.tr
https://bodrumteknekirala.com.tr
https://gocekteknekirala.com.tr
https://fethiyeteknekirala.com.tr
Keywords
Large vessel venous thromboembolism; Porcine animal model; Infusion catheter; Thrombolysis; Endovascular therapy
Abbreviations
VTE: Venous Thromboembolic Disease; HIV: Human Immunodeficiency Virus; DVT: Deep Vein Thrombosis; PE: Pulmonary Embolism; CTEPH: Chronic Thromboembolic Pulmonary Hypertension; BEC: Bashir™ Endovascular Catheter; r-tPA: recombinant tissue Plasminogen Activator; TEVF: Thrombus Extent and Venous Flow
Introduction
Venous Thromboembolic Disease (VTE) occurs in 1 to 2 persons per 1,000 people and accounts for ~180,000 deaths annually. It causes more deaths than breast cancer, motor vehicle accidents, and Human Immunodeficiency Virus (HIV) combined; it is the most preventable cause of in-hospital death [1]. Depending on the severity of the presenting symptoms and clinical status, patients are treated with systemic anticoagulation and/or advanced therapies like thrombolysis or thrombectomy (endovascular or open) [2]. Despite medical therapy with anticoagulation, nearly half of the patients with proximal lower extremity Deep Vein Thrombosis (DVT) develop post-thrombotic syndrome, and half of the patients with acute Pulmonary Embolism (PE) have functional limitations and impairment of quality of life commonly known as “post PE syndrome” [1,3].
Chronic Thromboembolic Pulmonary Hypertension (CTEPH) is another debilitating complication of large central PE with an incidence of 4%-16% [4]. Large animal models for the study of chronic pulmonary artery embolization have assisted in the creation of novel diagnostic tools and therapeutic strategies [5]. However, a need remains for a large animal model of acute thromboembolic disease involving large vessels, such as acute PE and Inferior Vena Cava (IVC) thrombosis.
VTE is a disease that exclusively affects humans [6]; since it does not occur naturally in any other animals, it necessitates the creation of a model in order to study the pathophysiology, squeal, and potential therapies for this condition. Previous models used to simulate VTE have been created in small animals by injuring the walls of veins with vascular clips or suture ligation, and by inducing thrombus formation through the introduction of various chemicals (e.g. ferrous chloride) or electrical current [6]. Murine models have provided a useful starting point in the evaluation of VTE but are of limited value due to their small anatomical size and the resulting small clot burden. As such, a Yucatan pig was selected due to its large vessels and because the coagulation and fibrinolytic systems are comparable to those of humans [7]. In this study, we have developed a novel animal model for acute large vessel VTE, in which the subject animal’s blood was allowed to coagulate in in- vitro for several days and then subsequently reintroduced into a large vessel.
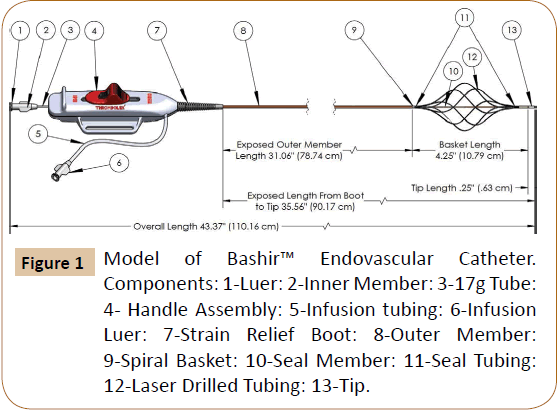
The use of catheter-based thrombus removal therapies is rapidly increasing in the United States for the treatment of both pulmonary embolism as well as proximal iliocaval DVT [2,8,9]. Current devices used for catheter-based therapy to treat acute VTE were developed using these small animal models [10]. In order to better address the treatment of large vessel venous thrombosis and thromboembolic disease, we have developed a novel infusion catheter called the Bashir™ Endovascular Catheter (BEC) (Figure 1).
Novel Infusion Catheter: The Bashir™ Endovascular Catheter is designed to be inserted into the clot via a percutaneous approach over an 0.018” guidewire. At its distal end it is comprised of six parallel mini-infusion catheters (infusion basket) that can be expanded via an actuator on the device handle. They expand in a spiral fashion to produce a large central channel to promote blood flow and allow the patient’s endogenous fibrinolytic agents to penetrate the clot and assist in dissolution. Furthermore, the miniinfusion catheters each contain eight laser-drilled longitudinally spaced holes to allow an exogenous thrombolytic agent (e.g. r-tPA) to be evenly distributed throughout the cross-section of the clot. In addition, the tip of the catheter can be used to monitor pressure and oxygen saturations in the pulmonary circulation.
Methods
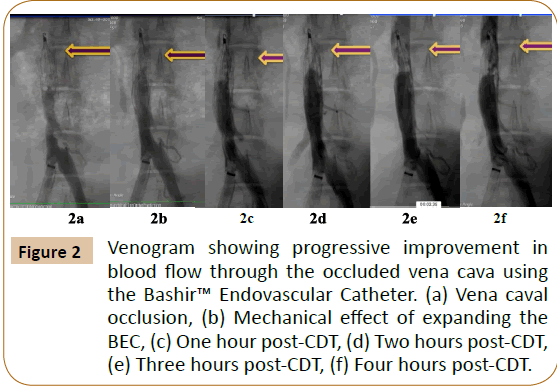
A venous blood sample (50 cc) was obtained from a Yucatan pig and coagulated in-in-vitro over a 12-hour period in a test tube at room temperature. The clot was then refrigerated for 4 days. Thereafter, the same animal was sedated, and the common femoral vein was accessed using a 14 French (F) sheath. Using a jugular venous access, a Denali® Vena Cava Filter was placed in the posterior cava of the swine. Under fluoroscopic guidance, contrast dye was injected to show brisk blood flow through the vena cava filter. Thereafter, the thrombus was injected into the posterior vena cava using a 20 cc syringe and a 12 F sheath. This led to a complete occlusion and cessation of blood flow across the posterior vena cava, which was documented by a venogram (Figure 2a).
Figure 2: Venogram showing progressive improvement in blood flow through the occluded vena cava using the Bashir™ Endovascular Catheter. (a) Vena caval occlusion, (b) Mechanical effect of expanding the BEC, (c) One hour post-CDT, (d) Two hours post-CDT, (e) Three hours post-CDT, (f) Four hours post-CDT.
After establishing the complete occlusion of the posterior vena cava, the swine was heparinized. An 0.018” guide wire was used to traverse the thrombus from the femoral vein. The BEC was advanced into the thrombotic vena cava over this wire and placed just proximal to the IVC filter. The infusion basket was expanded within the thrombus, which immediately led to a restoration of antegrade flow through the occluded cava (Figure 2b). An infusion of 0.04 mg/mL of Activase® (Tissue Plasminogen Activator; Genentech, South San Francisco, CA) solution was initiated at 6 mg/hr. Hourly evaluation of blood flow via fluoroscopic imaging was subsequently conducted for four hours (Figures 2c-2f). Finally, the infusion basket was collapsed and the device was retracted into the sheath. The clot and venous flow was assessed using an adaptation of the Thrombus Extent and Venous Flow (TEVF) grading system from the angiographic images as described in the National Venous Registry [11]. Our TEVF grades were defined as follows: Grade 0-patent vessel with normal flow; Grade 1-sub-segmental, non-occlusive thrombus; Grade 2-nonocclusive thrombus throughout length of the vessel; Grade 3-sub-segmental, occlusive thrombus; and Grade 4-an occlusive thrombus throughout length of segment.
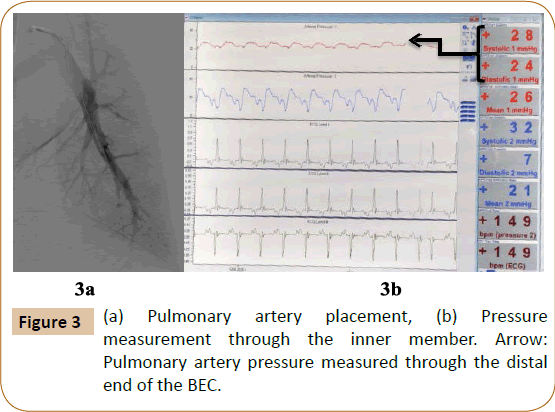
Pulmonary artery placement: A 70 cm sheath was advanced into the pulmonary artery and the BEC was positioned in one of the distal pulmonary artery branches to evaluate the feasibility of measuring PA pressures and mixed venous oxygen saturations through the distal end of the BEC (Figures 3a and 3b).
Results and Discussion
In this porcine animal model, we were able to successfully create a thrombotic occlusion of the posterior vena cava (TEVF grade-4) to evaluate the therapeutic effect of a novel infusion catheter.
Using this model, we were able to test the Bashir™ Endovascular Catheter; which successfully traversed the thrombus and, on expansion of the infusion basket, immediately led to initiation of antegrade flow across the occlusion (TEVF grade-2). We also achieved greater than 80% thrombolysis (TEVF grade-1) after four hours of catheter directed therapy-an improvement over current therapies [12,13]. Furthermore, we were able to use the BEC to measure the pulmonary artery pressures and mixed venous oxygen saturations, features that can be used to monitor Catheter- Directed Therapy (CDT) in acute pulmonary embolism.
The introduction of the thrombus into the posterior vena cava of our porcine model led to TEVF grade-4 occlusion, a finding further confirmed by the immediate development of collateral veins seen in the figure above (Figure 2a). Current devices for use in humans were developed using small animal models that are often unhelpful due to the small diameter and size of the vessels and the resulting small clot burden [6,10]. This novel model for venous thromboembolism will be very useful to evaluate the efficacy of various devices and catheters in development or ones already in use today.
Prior to initiation of thrombolysis, traversing the clot with the BEC and expanding the infusion basket produced immediate restoration of blood flow across the posterior vena cava (TEVF Grade 2) (Figure 2b). Mechanically passing a catheter through a thrombus allows the subject’s endogenous thrombolytic factors to permeate the clot to aid in lysis-a phenomenon commonly seen during percutaneous coronary intervention in patients with ST-elevation myocardial infarction. The spiral design of the BEC enhances the surface area across which the plasma can penetrate, thereby potentially decreasing the dose of exogenous thrombolytic therapy required.
Hourly venograms showed progressive improvement in venous flow and a decrease in the thrombus burden in the vena cava (Figures 2c-2f). Hourly results are expressed in Table 1 using TEVF grades and percentage clot burden to assess estimated thrombolysis. Each percentage was calculated based on previously published literature [14]. After four hours of thrombolytic therapy with Activase® infusion, we were able to achieve >80% thrombolysis. With standard of care therapy, the average time duration for thrombolysis in acute DVT is about 20 hours [12]. These findings suggest that the BEC has the potential to decrease thrombolytic dosage and duration, risk of bleeding, and ultimately hospital length of stay and healthcare costs.
| TEVF Grade | Clot burden | |
|---|---|---|
| Baseline | 0 | None |
| Post IVC Filter placement | 0 | None |
| Post thrombus injection | 4 | IVC below the filter full of thrombus |
| Post expansion of the infusion basket | 2 | Central channel with some flow |
| Post tPA infusion at one hour | 2 | 20% clot lysis without any further change in flow |
| Post tPA infusion at two hours | 2 | 30% clot lysis without any further change in flow |
| Post tPA infusion at three hours | 1 | 60% clot lysis with improved flow |
| Post tPA infusion at four hours | 1 | 80% clot lysis with brisk flow |
| Post device retrieval | 1 | 80% clot lysis with brisk flow |
Table 1: Hourly assessment of Thrombus Extent and Venous Flow (TEVF) grade for the large vessel venous thromboembolism model in comparison to the percentage of clot lysis.
The distal end of the BEC was successfully able to measure pulmonary artery pressures and mixed venous oxygen saturations (Figure 3). Conventionally, during thrombolytic therapy venography is performed to assess clot burden and titrate therapy. The BEC’s ability to monitor cardiac hemodynamics on a real-time basis in patients with acute pulmonary embolism will enable the operator to monitor progress and adjust the duration of thrombolysis accordingly, without the use of additional contrast or radiation [15,16].
The limitations of our study are as follows:
• When injecting the thrombus into the porcine model, there may have been fragmentation, which could have influenced the amount of time it took to achieve clot resolution.
• It is well established that in-vivo venous thrombosis leads to inflammatory changes within the vessel walls [17]; in our model however, after the thrombus was introduced, we promptly began testing our device and working towards clot resolution.
• Our model was tested using a higher hourly dose of Activase® than is currently used in clinical practice for catheter directed therapy.
Endovascular therapy has revolutionized the care of arterial thromboembolic diseases like cerebrovascular accidents and acute myocardial infarction and is now the standard of care in these patients [2]. In order to harness the potential of these endovascular therapies in the treatment of VTE we need to have well validated animal models that are anatomically similar to humans [8].
Conclusion
We have successfully created an animal model of an acute large vessel thromboembolism in the vena cava, and demonstrated that a therapeutic infusion catheter can be successful utilized in this model for thrombus removal. We believe that once this model is validated, it can foster rapid growth and development of thrombus reduction therapies in the treatment of VTE.
References
- Beckman MG, Hooper WC, Critchley SE, Ortel TL (2010) Venous thromboembolism. Am J Prev Med 38: S495-S501.
- Kohi MP, Kohlbrenner R, Kolli KP, Lehrman E, Taylor AG, et al. (2016) Catheter directed interventions for acute deep vein thrombosis. Cardiovasc Diagn Ther 6: 599-611.
- Kahn SR, Hirsch AM, Akaberi A, Hernandez P, Anderson DR, et al. (2017) Functional and exercise limitations after a first episode of pulmonary embolism. Chest 151: 1058-1068.
- Yang S, Yang Y, Zhai Z, Kuang T, Gong J, et al. (2015) Incidence and risk factors of chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. J Thorac Dis 7: 1927-1938.
- Aguero J, Hammoudi N, Bikou O, Fish KM, Zarragoikoetxea I, et al. (2018) chronic pulmonary artery embolization models in large animals. Methods Mol Biol 1816: 353-366.
- Diaz JA, Obi AT, Myers DD, Wrobleski SK, Henke PK, et al. (2012) Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol 32: 556-562.
- Olsen AK, Kornerup HA, Jespersen J, Marckmann P, Bladbjerg E-M (1999) The pig as a model in blood coagulation and fibrinolysis research. Scand J Lab Anim Sci 26: 214-224.
- Alkhouli M, Morad M, Narins CR, Raza F, Bashir R (2016) Inferior vena cava thrombosis. JACC Cardiovasc Interv 9: 629-643.
- Akhtar OS, Lakhter V, Zack CJ, Hussain H, Aggarwal V, et al. (2018) Contemporary trends and comparative outcomes with adjunctive inferior vena cava filter placement in patients undergoing catheter-directed thrombolysis for deep vein thrombosis in the United States. JACC Cardiovasc Interv 11: 1390-1397.
- Lin PH, Chen C, Surowiec SM, Conklin B, Bush RL, et al. (2001) Evaluation of thrombolysis in a porcine model of chronic deep venous thrombosis: An endovascular model. J Vasc Surg 33: 621-627.
- Porter JM, Moneta GL (1995) Reporting standards in venous disease: an update. International consensus committee on chronic venous disease. J Vasc Surg 21: 635-645.
- Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, et al. (2017) Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med 377: 2240-2252.
- Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis: Analysis from a stratified multicenter randomized trial. Circulation 139: 1162-1173.
- Ouriel K, Greenberg RK, Green RM, Massullo JM, Goines DR (1999) A volumetric index for the quantification of deep venous thrombosis. J Vasc Surg 30:1060-1066.
- Andreucci M, Faga T, Serra R, De Sarro G, Michael A (2017) Update on the renal toxicity of iodinated contrast drugs used in clinical medicine. Drug Healthc Patient Saf 9: 25-37.
- Gilbert ES (2009) Ionising radiation and cancer risks: What have we learned from epidemiology? Int J Radiat Biol 85: 467-82.
- RoumenÃÆâÃâââ¬ÃâÃÂKlappe EM, Janssen MCH, van Rossum J, Holewijn S, van Bokhoven MMJA, et al. (2009) Inflammation in deep vein thrombosis and the development of postÃÆâÃâââ¬ÃâÃÂthrombotic syndrome: A prospective study. J Thromb Haemost 7: 582-587.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences