Preclinical Investigation of a 2nd Generation DermaPort™ Ported Vascular Access System (PVAS)
Abram D Janis, Thomas J Lobl and Buzz Moran
DOI10.21767/2573-4482.19.04.17
Abram D Janis1, Thomas J Lobl2* and Buzz Moran3
1Formerly DermaPort, Inc., Walnut, CA, USA
2Formerly DermaPort, Inc. and Alfred Mann Institute, University of Southern California, Los Angeles, CA, USA
3Formerly DermaPort, Inc. and Moran Medical Device Consulting, Santa Barbara, CA, USA
- *Corresponding Author:
- Thomas J Lobl
Lobl TJ, Alfred Mann Institute for Biomedical Engineering
University of Southern California, Los Angeles
California, USA
Tel: (213) 821-1745
E-mail:Tom.Lobl@USC.edu
Received date: August 03, 2019 Accepted date: September 05, 2019 Published date: September 12, 2019
Citation: Janis AD, Lobl T J, Moran B.Preclinical Investigation of a 2nd Generation DermaPort™ Ported Vascular Access System (PVAS). J Vasc Endovasc Therapy 2019, Vol.4 No.3:17
Abstract
Introduction: The DermaPort™ Ported Vascular Access System (PVAS) was developed to improve central line access and reduce infections in hemodialysis while permitting catheter repositioning or replacement without disruption of the ingrown port. The PVAS port is comprised of a percutaneous titanium conduit with a subcutaneous titanium mesh ingrowth cuff and a disengageable silicone brake. Feedback from the clinical trial revealed that replacement of the peel away sheath (Gen1) with a dilating housing (Gen2) would be an improvement. Objective: Design and validate in vivo a Gen2 design that replaces the user-dependent peel away sheath. Methods: Three candidate housing designs and then three potential mesh cuff positions for the optimized Gen2 dilating housing without a sheath were screened in an acute porcine insertion model. These designs were compared to Gen1 with a sheath and a polyester-cuffed catheter. Next, the selected design and three potential mesh cuff positions were further tested in chronic 6-week and 4-week chronic rabbit studies, respectively. Both chronic studies histologically characterized mesh ingrowth and marsupialization in comparison to Gen1 and polyester-cuffed catheter controls. Results: Insertion of the Gen2 housing with a 10° dilating distal end was determined to be the most similar to Gen1 in the porcine model. The 4 and 6-week rabbit implant study showed a trend towards greater down growth for the deeper mesh designs and no observation of marsupialization. There were no significant differences in connective tissue ingrowth between the two PVAS housing designs or the mesh positions in the six week study, which was more mature and less inflammatory than in polyester-cuffed controls. Conclusions: These data provide support for the further development of the Gen2 PVAS in response to clinical feedback on the Gen1 design.t.
Keywords
Hemodialysis; Ported port; Tissue integration; Infection; Vascular access system
Introduction
Safe chronic percutaneous access to the body is required for an increasing number of medical devices and procedures including catheters for kidney dialysis (both peritoneal and hemodialysis). Chronic hemodialysis is associated with two key clinical problems: infections and the need to exchange or reposition the catheter as a result of thrombosis, diminished flow or infection. These key clinical problems are not well addressed by currently available tunneled central venous access catheters [1].
Current hemodialysis catheters rely on a subcutaneous polyester cuff to anchor the catheter in the tunnel and following soft-tissue ingrowth, prevent the passage of bacteria into the proximal tunnel. Disadvantages include lack of potential for easy repositioning and convenient removal or exchange. The cuff may also become detached from the catheter body during catheter removal [2]. In addition, the cuff to catheter tip length is fixed and cannot be changed before or following implantation to accommodate patient-specific anatomy. Any modification of the catheter position with an ingrown cuff requires surgical dissection from the subcutaneous tunnel. The resultant replacement with a new catheter and cuff will again take at least 2-3 weeks for ingrowth to provide the necessary sterile barrier and healing along with the associated risks from a scarred tunnel. At the venous access site, this can also eventually result in depletion of the patient’s central venous access sites [3]. Preservation of central veins is a fundamental tenet for management of chronic hemodialysis patients. Despite these limitations in standard of care, catheter exchange remains a necessary and common event
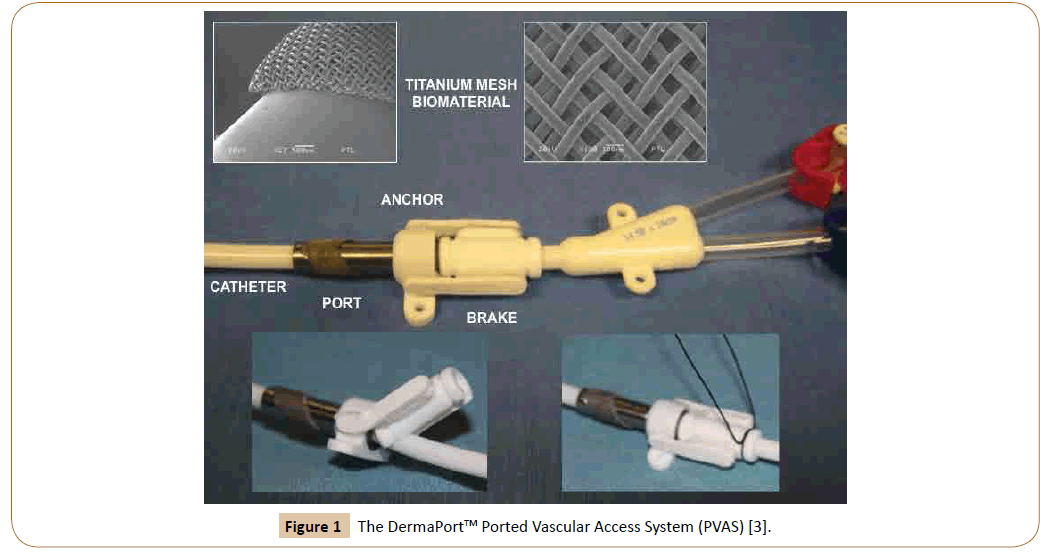
The DermaPort™13 Ported Vascular Access System (PVAS) (Figure 1) [4] (IRRAS USA. San Diego CA) was designed to improve central venous access and reduce infections in hemodialysis by combining a tissue-integrated, percutaneous conduit with a chronic tunneled hemodialysis catheter. This design, additionally, eases catheter placement, repositioning and exchange while preserving the fixation and infection-resisting properties of a cuff. The PVAS is comprised of a percutaneous, cylindrical, titanium (Ti) conduit surrounded by a subcutaneously positioned Ti mesh, ingrowth cuff. A peel away sheath eases the atraumatic subcutaneous placement of the cuff through an undersized 0.29” incision. The 14.5 F dual lumen catheter is then anchored to the conduit by a releasable silicone brake held in place with a looped suture. This decoupling of the tissue ingrowth interface from the catheter permits more precise tip placement and catheter repositioning and exchange without the need for a new cuff ingrowth. Once complete, the tissue ingrowth into the Ti mesh provides the necessary sterile barrier [4].
Figure 1: The DermaPortTM Ported Vascular Access System (PVAS) [3].
The catheter exchange procedure for the PVAS
To exchange or reposition the catheter, a guidewire is threaded through one lumen to the right atrium. The stay suture loop is cut and the silicone brake is lifted from the catheter (Figure 1), enabling retraction for tip repositioning or removal for exchange. For exchange, a new catheter is then advanced over the guide wire and the brake is re-engaged. Finally, a new suture is looped around the brake to prevent unintentional disengagement.
Reports describing the design, development and in vitro, and in vivo testing as well as clinical performance of the 1st generation PVAS (Gen1) have recently been published [4,5]. Human factors feedback from the 38 subject clinical trial revealed that the performance of the dilating peel away sheath used during implantation was user-dependent, awkward and could be improved in future designs. In responding to this feedback, we have modified the design of the PVAS to add a dilating bevel onto the housing. Further, the implantation procedure has been revised to make it more convenient for the surgeon working under sterile conditions, simplified the implantation surgery procedures and reduced the number of component parts to the PVAS system. Here we describe the improved design and in vivo validation of a 2nd generation PVAS (Gen2) design that obviates the need for a peel-away sheath and simultaneously addresses the user suggestions.
Methods
response to user feedback, several design modifications were evaluated to eliminate the need for the peel away sheath of Gen1. The Ti mesh (cuff) positions and angles were also evaluated to evaluate the entry angles and ingrowth of the PVAS and the skin. From this work, three candidate Gen2 PVAS designs and cuff positions for the dilating housing were selected and compared to Gen1 with sheath in an acute porcine insertion model. Then, the most promising housing design (without a peel away sheath) and three potential mesh cuff positions were tested in a chronic rabbit implant models at 4 weeks then in a second model at 3 and 6 weeks, respectively. Both chronic rabbit studies histologically characterized the mesh ingrowth and marsupialization in comparison to Gen1 PVAS and polyester-cuffed catheters. The porcine study was performed at Med Vice Inc. (Rancho Cucamonga, CA) and rabbit studies were performed at Biodevelopment Associates (Mountlake Terrace, WA). All animal housing and care conformed to standards published in the “Guide for the Care and Use of Laboratory Animals” NIH Publication No. 86-23 and were under the supervision of the facility IACUC. Histology was performed at Wasatch Histo Consultants (Winnemucca, NV) and histomorphometry was performed by BioGenetics Research Laboratories, Inc. (Greenbank, WA).
Porcine insertion screening study
Two pigs (130 and 150 lbs) were sedated, intubated and anesthetized with isofluorane. The ventral inguinal skin was depilated and cleaned. In bilaterally paired sites, two users graded the three Gen2 designs using visual analog scales (VAS) for ease of insertion, tissue integrity at the insertion site and mesh integrity following insertion. The Gen1 PVAS with sheath was used to calibrate each user on each pig. Fourteen and twentynine insertions and evaluations were performed on the first and second pigs, respectively, on one day.
Pilot chronic rabbit implant model
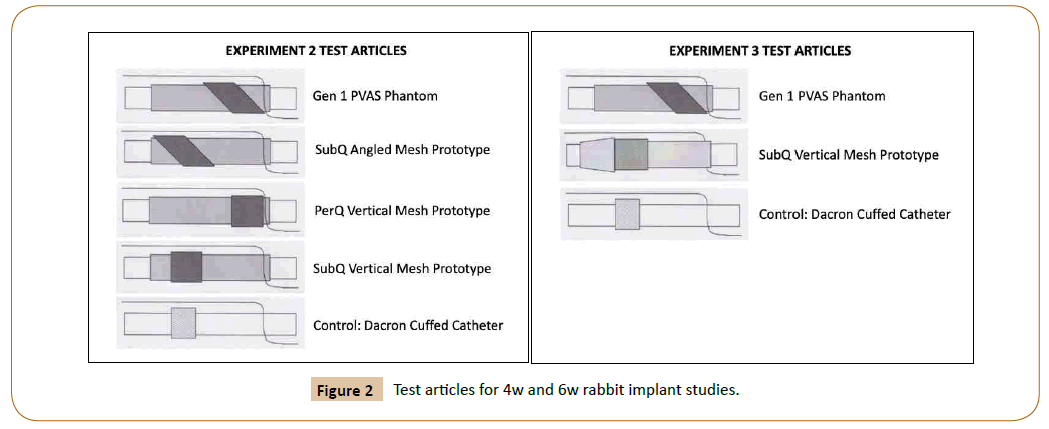
Four female New Zealand (NZ) white rabbits (3-5 kg) were sedated, intubated and anesthetized with isofluorane. The dorsa were shaved and prepped for sterile surgery. One control polyester-cuffed catheter and three of four Gen2 PVAS implants (Figure 2) were implanted in pairs parallel to the spine in each rabbit (N=3 test articles, N=4 controls). Animals were recovered and housed until euthanasia and explantation at 4 weeks (4 w). Implants were excised en bloc with surrounding tissue and fixed in 4% neutral buffered formalin.
Definitive chronic rabbit implant model
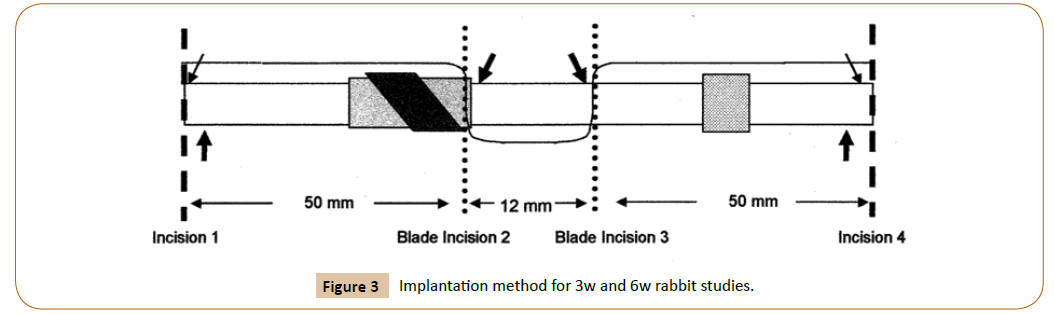
Ten female NZ White rabbits (3-5 kg) were sedated, intubated and anesthetized with isofluorane. Control polyester-cuffed catheters, Gen1 PVAS with angled mesh and Gen2 PVAS with dilating housing with 90° mesh (Figure 3) were implanted in pairs parallel to the spine in each rabbit. Forty implants were deployed (N=12 controls and N=14 of each test article). Animals were recovered and housed until euthanasia and explantation at 3 weeks (N=5 rabbits) and 6 weeks (N=5 rabbits). Implants were excised en bloc with surrounding tissue and fixed in 4% neutral buffered formalin.
Histopathology
Fixed implants and tissue were embedded in methyl methacrylate. Two longitudinal wafers centered on the long axis were sectioned. The remaining tissue blocks were glued back together and serial perpendicular sections were cut. Sections were polished, mounted to slides and stained with H&E and Wasatch trichrome with aniline blue counterstain. The pathologist scored the capsule quality, capsule thickness, interstitial tissue quality, and tissue response. The epidermal down growth was measured on each device.
Statistics
One-way ANOVA with Dunn’s pairwise post-hoc comparisons was used to compare the data. Statistical significance was determined at P<0.05.
Results
Porcine insertion screening study
Insertion of the Gen2 test article with a 10° dilating distal end was determined to be the most similar to Gen1 PVAS with a peel away sheath in the porcine model. No statistically significant differences were found for the three endpoints, likely due to the semi-quantitative nature of the study, however this test article trended most similar to the Gen1 PVAS with sheath.
Pilot chronic rabbit implant model
The 4 w rabbit implant study showed a trend towards greater down growth for the deeper mesh designs and no observation of marsupialization past the mesh cuff. The fibrous connective tissue surrounding the four test articles with titanium mesh cuffs was more mature, with more vascular tissue and less inflammatory cells compared to the polyester-cuffed controls. There were no statistical differences between the four test articles. Based on these results and the results of the porcine screening study, the Gen2 test article with a 10° dilating distal end was selected for further testing in the definitive chronic model.
Definitive chronic rabbit implant model
The infiltration of fibrovascular connective tissue into the titanium mesh cuffs of the Gen1 PVAS and Gen2 PVAS was more complete (94-100% at 3 w and 6 w) than it was in the polyestercuffed controls (78% at 3 w and 88% at 6 w). The voids of the titanium mesh were nearly completely filled with vascular fibrous tissue by 3 w and by 6 w there were fewer macrophages and an increased density of fibroblasts with early collagen fiber deposition. Mean percent ingrowth for Gen1 PVAS implants at 3 and 6 weeks were significantly greater than controls at 3 w, as were Gen2 PVAS implants at 6 w (P<0.05, One-way ANOVA with Dunn’s pairwise comparisons). Mean connective tissue ingrowth for the two test PVAS phantoms consistently trended higher than control polyester-cuffed catheter phantoms. There were no significant differences between the two PVAS phantom types. The pathologist graded the down growth of the epidermis from the skin surface along the shaft of the device. The Gen2 design had the lowest average scores (1.00 and 1.29 at 3 w and 6 w respectively) compared to the Gen 1 PVAS (1.33 and 1.71 at 3 w and 6 w, respectively) and the polyester-cuffed controls (1.80 and 1.50 at 3 w and 6 w, respectively). No statistically significant differences were found for these parameters among the Gen2 PVAS and the Gen1 PVAS designs confirming the equivalence of the improved design with the Gen1 DermaPort PVAS (Table 1).
Table 1: Three and 6-week explant histomorphometry.
| Connective Tissue Ingrowth (%) | Epidermal Down growth | ||
|---|---|---|---|
| 3 weeks | Polyester-cuffed | 78 | 1.80 |
| Gen1 PVAS | 98.6 | 1.33 | |
| Gen2 PVAS | 93.6 | 1.00 | |
| 6 weeks | Polyester-cuffed | 88 | 1.50 |
| Gen1 PVAS | 100 | 1.71 | |
| Gen2 PVAS | 100 | 1.29 |
Discussion
There are two key clinical problems associated with hemodialysis: infection and catheter exchange or repositioning [6,7]. Current hemodialysis catheters feature a polyester-cuff attached to the catheter as a site for tissue ingrowth and to provide a sterile barrier. The disadvantage of this method of anchorage is that it non-reversibly fixes the catheter within the subcutaneous tunnel [8-11]. This fixed cuff anchor prevents catheter adjustment following implantation when suboptimal for a patient’s anatomy, repositioning during use and convenient removal and exchange [12]. A solution addressing these issues is the combination of the hemodialysis catheter system with the capability to provide an infection-resisting seal between the catheter and the skin. The DermaPort™ PVAS (IRRAS, San Diego, CA) was designed to combine a tissue-integrating percutaneous port with the infection-resisting properties of a cuffed long-term hemodialysis catheter. The results of the Gen1 studies in animals indicate that the system provides superior tissue integration performance coupled with infection–resisting slidability, allowing reposition and exchange of an indwelling catheter thereby permitting stable ported access [4]. Based on these results the Gen1 system was used in a clinical trial. Results from the thirty-eight subject clinical study in hemodialysis patients using the Gen1 PVAS demonstrated 100% technical success with the implantation site demonstrating early tissue incorporation after 2 weeks and full incorporation within 4 weeks [5]. In summary, the DermaPort™ Gen1 successfully enabled 31 catheter exchanges and 10 repositions through the port without dissection in 18 patients with nine repositions (90%) performed at bedside without fluoroscopy. There were no SAEs in 12,100 catheter days of use and zero infection-related explantations [5].
Human factors feedback from this clinical trial revealed that the performance of the dilating peel away sheath used during implantation was user-dependent and awkward in the surgical implantation procedures. It was suggested by the clinical staff that the implantation procedure would be improved if the dilating peel away sheath was replaced with a device design that did not require it. The Gen2 design is a response to this feedback. The in vivo data presented here provide support for the potential further development and investigation of a Gen2 PVAS with improved human factors in response to this clinical user feedback on the Gen1 design. The improved housing design includes a beveled edge to the DermaPort™ PVAS to enable easier dilatation and insertion into the percutaneous exit site without the need for a dilating, disposable sheath. This reduces the number of parts in the system, reduces the surgical time and simplifies the implantation procedures.
Conclusion
The animal studies demonstrated that there were no significant differences in connective tissue ingrowth between the Gen1 PVAS and Gen2 designs. Additionally, in the Gen2 design with a 10° dilating housing and 90° mesh showed the tissue ingrowth trended higher and more mature with less inflammatory cell infiltration than the polyester-cuffed controls in the 3 w and 6 w study. The Gen2 design does not require the use of a dilating peel away sheath to percutaneously implant the PVAS.
It should be noted that the observed catheter infection rate in the clinical trial of the Gen1 device was 0.33/1000 days, which was lower than historical outcomes with traditional tunneled dialysis catheters [5]. As the tissue ingrowth and histopathological findings are similar between the two designs it is anticipated the safety of the Gen2 design will be comparable to the Gen1 design while offering a more convenient implantation procedure and a simpler product design. Based on the observations in these studies, these improvements to the design are a significant improvement to the DermaPort™ PVAS product.
Acknowledgements
The authors thank DermaPort™, Inc. for financial and material support in developing the product, the Alfred Mann Institute for Biomedical Research at the University of Southern California and IRRAS AB for financial support. The authors were paid consultants to DermaPort™, Inc. The materials in this paper was presented, in part, as a poster at the ASAIO 65th Annual Conference, San Francisco, CA 94102, June 26-29, 2019.
Disclosure
All authors are former DermaPort™ employees and/or consultants.
References
- Sequiera A, Naljayan M, Vachharajani TJ (2016)Vascular access guidelines: Summary, Rationale, and Controversies. Tech Vasc Interv Radiol20: 2-8.
- Kohli MD,Trerotola SO, Namyslowski J,Stecker MS,McLennan G, et al. (2001) Outcome of polyester cuff retention following traction removal of tunneled central venous catheters. Radiology 219: 651-654.
- Pieters PC, Tisnado J, Mauro MA (2003) Venous Catheters: A Practical Manual. Book reviews 27: 217-218.
- Lobl TJ, Janis AD, Moran B (2019) DermaPort: A novel ported vascular access system for hemodialysis. ASAIO J.
- Rajan DK, Moran B, Lobl TJ, Asch MR, Steele AW, Lok CE (2018) A prospective clinical study of a percutaneous vascular access system for hemodialysis catheters. Cardiovasc Intervent Radiol 41: 1513-1519.
- Sequeira A, Naljayan M, Vachharajani TJ (2016) Vascular access guidelines: Summary, rationale, and controversies. Tech Vasc Interv Radiol20: 2-8.
- Work J (2001) Chronic catheter placement. Semin Dial14: 436-440.
- Janne d’Othée B, Tham JC, Sheiman RG (2006) Restoration of patency in failing tunneled hemodialysis catheters: A comparison of catheter exchange, exchange and balloon disruption of the fibrin sheath, and femoral stripping. J Vasc Interv Radiol17: 1011-1015.
- Robinson D, Suhocki P, Schwab SJ (1998) Treatment of infected tunneled venous access hemodialysis catheters with guidewire exchange. Kidney Int53: 1792-1794.
- Tanriover B, Carlton D, Saddekni S, Hamrick K, Oser R, et al. (2000) Bacteremia associated with tunneled dialysis catheters: Comparison of two treatment strategies. Kidney Int 57: 2151-2155.
- Weijmer MC, Vervloet MG, Ter Wee PM (2004) Compared to tunneled cuffed haemodialysis catheters, temporary untunnelled catheters are associated with more complications already within 2 weeks of use. Nephrol Dial Transplant19: 670-677.
- Butterly DW, Schwab SJ (2001) Catheter access for hemodialysis: An overview. Semin Dial14: 411-415.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences