Thoracic Endovascular Aneurysm Repair (TEVAR) for Ruptured Thoracic Aortic Aneurysms
Furlough CL and Eskandari MK
DOI10.21767/2573-4482.100046
Furlough CL and Eskandari MK*
Division of Vascular Surgery, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
- *Corresponding Author:
- Mark K Eskandari
Division of Vascular Surgery
Northwestern University Feinberg School of Medicine
676 N St Clair Street, Ste 650
Chicago, IL 60611, USA
Tel: 312-926-7775
E-mail: jgoldste@nm.org
Received Date: April 19, 2017; Accepted Date: April 24, 2017; Published Date: May 02, 2017
Citation: Furlough CL, Eskandari MK. Thoracic Endovascular Aneurysm Repair (TEVAR) for Ruptured Thoracic Aortic Aneurysms. J Vasc Endovasc Surg. 2017, 2:14. doi: 10.21767/2573-4482.100046
Abstract
Background: Rupture in Descending Thoracic Aortic Aneurysms (rDTAA) is a fatal complication. Open repair has long been the traditional standard of treatment; however, thoracic endovascular repair (TEVAR) has become increasingly popular to treat this complication. Even with continued surgical advances, open repair (OR) remains associated with high morbidity and mortality. The introduction of TEVAR and its common use in the treatment of rDTAA has raised the question of the possible superiority of one over the other.
Purpose: This review discusses specific considerations in rDTAA repair including initial management, complications, and results of OR and TEVAR.
Conclusion: Despite lacking evidence, TEVAR is often first choice treatment for rDTAA and leads to early survival; however, it has specific device related complications that affect long term outcomes.
Keywords
Rupture; Aneurysm; Thoracic aorta; Endovascular
Introduction
Repair of Ruptured Descending Thoracic Aortic Aneurysms (rDTAA) remains a challenging surgical undertaking. Although rupture is rare, when it occurs it is often a lethal event. Traditionally the standard of repair for rDTAA has been open surgical repair requiring thoracotomy, aortic cross-clamping, and cardiopulmonary bypass. However, while the standard, these open procedures are accompanied by significant perioperative complications and thus in themselves carry a high morbidity and mortality. Thoracic Endovascular Aneurysm Repairs (TEVAR), first described in 1994, and offered an alternative for a less invasive approach [1]. Since its introduction, TEVAR has been widely applied and accepted as a first-line method of treatment for various thoracic aortic pathologies including dissection, blunt traumatic aortic injury, and degenerative aneurysm. In the elective setting, TEVAR is praised for its well-documented benefits of decreased operative time, decreased blood loss, and perioperative declines in morbidity and mortality when compared with open surgical repair [2-5]. In the emergent setting, its rapid deployment and minimally invasive qualities make TEVAR particularly appealing. Little data exists, however, specifically regarding outcomes of emergent TEVAR for ruptured aneurysms of degenerative atherosclerotic etiology. The aim of this paper is to review the specific considerations associated with TEVAR for rDTAA.
Natural History
The natural history of aneurysmal degeneration of the thoracic aorta is challenging. Aneurysms of the descending thoracic aorta occur with an estimated incidence of 5.0 per 100,000 people per year with ruptures occurring at a nearly equal incidence in the population [6-8]. Risk of rupture is affected by multiple factors; however, the single most important factor is maximum aortic diameter. Longitudinal studies show that generally TAAs enlarge at a rate of 3 mm/year with faster rates of growth seen in larger aneurysms [6,9]. However, rate of expansion is at times unpredictable, making repair essential when a diameter of 6 cm is reached [9-11]. Over 5 years, longitudinal evaluation of rupture risk approximated 16% for aneurysms measuring 4-5.9 cm and 31% for >6 cm [6,12]. Other significant contributors to increasing risk of rupture include presence of Chronic Obstructive Pulmonary Disease (COPD), advanced age, and presence of chronic back pain. The majority of patients who experience rupture of the thoracic aorta do not survive to present to a hospital. Of those that do, few have a successful outcome. Reported mortality rates are notably very high, ranging from 25% to 45% [2]. Grossly, national estimates, however, do not make a distinction between ruptures related to traumatic injury, dissection, mycotic or atherosclerotic origin. This matters, as prognosis is closely related to etiology of rupture. Aneurysms associated with dissection had the highest rates of rupture ahead of atherosclerotic (degenerative) aneurysms and aneurysms in patients with connective tissue disease. Non-traumatic aortic ruptures were found to have significantly greater mortality than traumatic ruptures, highlighting the influence of severe risk factors in patients with non-traumatic ruptures [13]. These patients tended to be older with complex pre-existing co-morbid conditions to contribute to their overall outcomes.
Patient selection
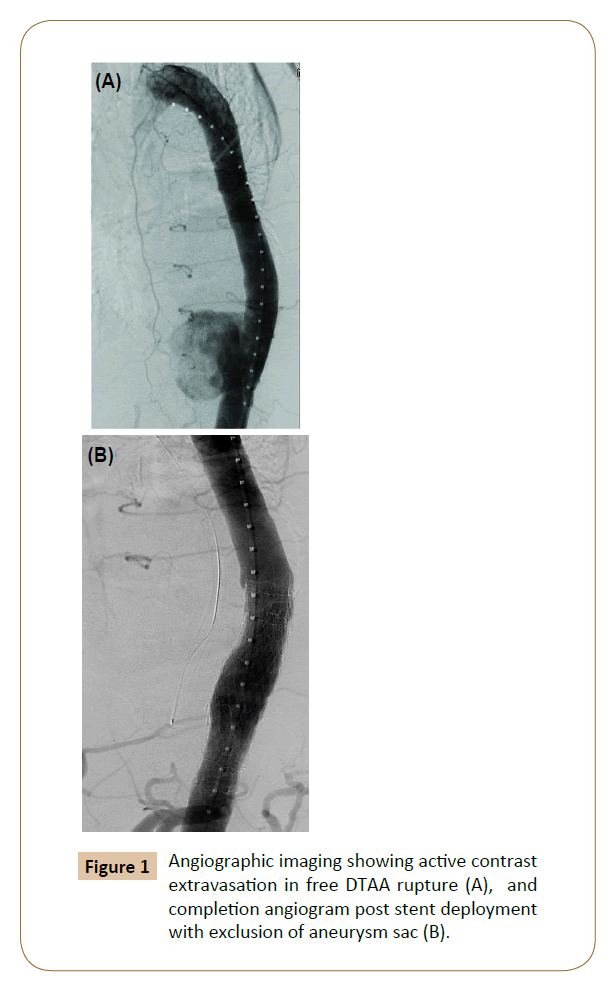
The gold standard therapy for elective or emergent descending thoracic aneurysms has traditionally been open repair regardless of etiology [2-5]. Early outcome data has demonstrated that TEVAR has had notable early success in treating ruptured DTAA. Jonker et al. published a meta-analysis comparing TEVAR vs open repair, which showed that TEVAR is associated with a lower 30-day mortality rate in rDTAA [8]. The benefit of TEVAR is that it extends an option for repair to patients that previously were considered too high-risk for open repair, primarily elderly patients and those with complex co-morbidities. Also, the ability to perform TEVAR expeditiously decreases operative time and minimizes blood loss making it preferable to open repair in a presentation of rupture. The largest concern, however, is which patients in these categories are actually eligible for endovascular repair and which patients presenting with rupture should be treated with an endoluminal approach (TEVAR) (Figure 1).
Assessment of anatomic suitability
The first step in directing patients to endovascular versus open repair is the ability to obtain adequate cross-sectional imaging either by Computed Tomography (CT) or magnetic resonance (MR) imaging. The ability to expeditiously obtain this imaging is paramount to minimizing the time to making this decision. Computed Tomography Angiography (CTA) is the primary imaging modality used to evaluate for acute aortic pathologies such as aneurysm rupture. The imaging allows the team to quickly obtain cross-sectional imaging with thin slices to visualize the details of the aneurysm, which is necessary to determine graft suitability. Adequate imaging must include the vasculature extending from supra-aortic to the common femoral vessels (access vessels). Coselli and Gopaldas emphasized that at each center efficient management of aortic rupture involves coordination of multiple levels of personnel from the emergency room to the surgical team [2]. Centers should have an established structured plan of rapidly obtaining CT imaging whenever thoracic aortic rupture is suspected as well as immediate notification of surgical teams with endovascular capability and a team capable of performing open repair if endovascular management is not feasible. Thorough knowledge of the patient’s vasculature including patency of vertebral arteries, measurement of aortic diameter, and aneurysm proximity to the Left Subclavian Artery (LSA), access vessel size, and arch angulation are all paramount to determining anatomic suitability and thus preparing the patient for endovascular repair. Aneurysm diameters too wide for commercially available devices, tortuous or highly calcified access vessels, and aneurysms with necks too short to allow for adequate seal exclude patients from candidacy for endovascular repair. Use of CTA provides early detection of these characteristics and allows rapid decision-making for endovascular versus open surgical intervention.
Surgical and Anesthetic Considerations
Control of hemorrhage
In emergency situations, obviously, formal preoperative evaluation and optimization is foregone in order to minimize time between patient arrival, diagnosis, and intervention. The main therapeutic goal becomes achieving hemodynamic stability with maintenance of vital organ perfusion accomplished by permissive hypotension. This concept involves maintaining a systolic blood pressure of 70-80 mmHg and avoiding aggressive volume resuscitation to pressures >100 mmHg [14]. The idea supporting this concept is that aggressive volume replacement prior to achieving surgical control contributes to further hemorrhage and higher mortality. While proximal aortic control is achieved by aortic cross clamping or, in percutaneous repair, with balloon occlusion and has been successfully employed for the management of ruptured abdominal aortic aneurysm, balloon occlusion is less technically feasible in many instances of rDTAA.
Selection of type of anesthesia
Emergency TEVAR has been performed under local, regional, and general anesthesia. No consensus guidelines exist to recommend one technique over another and no large comparative studies are available to demonstrate superiority of any technique. The choice of anesthesia is unique and related to various patient factors such as degree of hemodynamic instability, pre-existing co-morbidities, and compliance. Additionally, anesthesia and surgical team comfort/preference is varied in different centers. In the studies reviewed, the majority of repairs were performed under general anesthesia. Percutaneous repairs under local anesthesia have been reported with surprising frequency and have even been recommended in patients deemed higher risk due to co-morbid conditions and hemodynamic instability, as local anesthesia can minimize the blood pressure fluctuations encountered with induction in general anesthesia [14]. Patient cooperation, however, is essential to safe and precise stent graft deployment and in time of acute pain and distress may be unable to be attained with only local anesthesia. General anesthesia also must be considered when additional interventions, carotidsubclavian bypass for example, are required.
Extent of coverage
When compared with isolated traumatic aortic injury, longer coverage zones will be necessary to completely exclude the aneurysm sac in rupture. This may lead to coverage of the left subclavian artery proximally or the celiac artery distally for complete exclusion. Whereas the option to perform a staged carotid subclavian bypass or transposition exists, in emergent repair with TEVAR requiring LSA coverage, often these patients are managed expectantly and revascularization is later performed as needed if the patient becomes symptomatic. Several studies have shown that patients do tolerate LSA coverage well and expectant management is reasonable in emergent cases [12,15]. Guidelines do exist, however, establishing a particular set of circumstances that mandate revascularization even with emergent repairs [16]. These include prior left internal mammary artery to coronary bypass, occluded or absent right vertebral artery, dominant left vertebral, or long segment coverage of the thoracic aorta (>25 mm).
Neuroprotective measures
One of the many complications of thoracic aortic repair is spinal cord ischemia. When possible, steps are taken preoperatively to reduce its risk, namely through cerebrospinal fluid drainage. Rationale for this strategy is based on the fact that spinal cord perfusion pressure can be increased by increasing the mean arterial pressure or by use of a lumbar drain to decrease it [14,17,18]. No consensus formally exists; however, patient presentation often determines the ability to institute this neuroprotective measure. In a series by Girardi et al. all patients who presented with contained ruptures and normal hemodynamics had cerebrospinal fluid drainage instituted pre-procedure [11]. However, in cases such as ruptures where the patients were relatively unstable, emergent TEVAR was performed and placement of a lumbar drain was performed post-procedure. Peri-operative placement of Cerebrospinal Fluid (CSF) drain is recommended as a spinal cord protective strategy in patients with risk factors for spinal cord ischemia. In some centers, lumbar drain placement was done post-operatively only if patients became symptomatic with evidence of spinal cord ischemia and paraplegia [2,14,17,18]. CSF drains themselves are not without substantial risk. Complications include headaches, spinal hematomas, meningitis, and persistent CSF leaks, all of which can develop to become notable adverse neurologic events.
Complications of TEVAR
Spinal cord ischemia
Paralysis and paraplegia are other dreaded complications of thoracic endovascular repair. Data suggest an incidence of these complications of 2-6% after elective TEVAR and an increase with emergency surgical procedures particularly for aortic ruptures and dissection [19]. It is a devastating complication often associated with hypotension in the peri-operative period [14]. Contributors include coverage of the left subclavian artery, prior or concomitant AAA repair, pelvic occlusive disease, renal failure, or long segment coverage of the thoracic aorta where intercostals supplying the spinal cord may be compromised [14,18,19].
Peri-procedural stroke
TEVAR for rDTAA is associated with considerable risk of stroke and contributes significantly to peri-procedural mortality for patients. The presumed mechanism is thought to be embolization of atherosclerotic plaque from manipulation of catheters and wires in a diseased aortic arch [4,20]. The exact risk of stroke is not completely clear, given the low incidence of rDTAA; however, rupture-specific contributing risk factors include the duration of procedure and profound hypovolemia from blood loss affecting adequate cerebral perfusion [20]. Risk of stroke also increases significantly with extension of coverage into zone 0 or 1 (proximal arch to left carotid) although it is not clearly defined why this occurs. Procedure-related stroke occurs at an increased rate after emergency TEVAR when compared with elective TEVAR; however, still higher is the incidence of stroke after emergent open procedures thus allowing TEVAR to remain a preferred treatment for rDTAA in patients deemed anatomically suitable [20].
Predictors of mortality
With the paradigm shift toward endovascular first treatment of thoracic aortic aneurysmal disease, surgeons have noted improvements in early clinical outcomes, mortality, and morbidity with TEVAR compared to open repair. Patients with descending thoracic aortic disease often present with significant risk factors such as advanced age or presence of aortic unrelated co-morbid conditions that increase open surgical repair risks. A large study of 923 patients who underwent intervention for rDTAA noted common baseline patient characteristics of chronic peripheral vascular disease, cerebrovascular disease, congestive heart failure, chronic renal disease, and COPD, all of which were found with greater frequency in the groups who underwent TEVAR [21]. Thus, late mortality seen in TEVAR is often greatly affected by the presence of these co-morbidities, which not only influence open surgical risks but independently influence overall long-term survival.
Long-term results of TEVAR and more specifically TEVAR for rDTAA are not yet widely available; however, the move behind this paradigm shift is supported by significant decreases in immediate mortality and early complication rates [2,3,8,22]. Open repair itself exists as an independent predictor of mortality. It requires a posterolateral thoracotomy using extracorporeal perfusion support. In extensive cases, hypothermic circulatory arrest may be necessary. Operative time is incomparably longer and blood loss is substantially greater than in endovascular repair. Open repair carries higher incidences of systemic complications such as acute kidney injury (AKI) and respiratory failure, which are both markers of increased mortality after aortic surgery (Figure 1) [23].
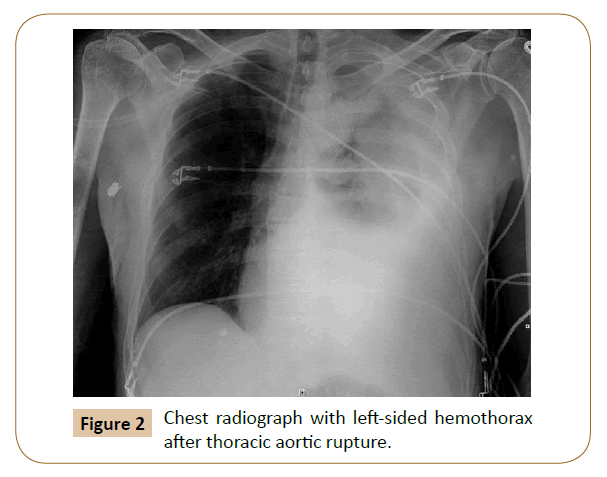
Other independent predictors exist with regard to repair of rDTAA-hemothorax and hypovolemia (Figure 2). Hemothorax at presentation is a strong predictor of mortality [3]. It is a marker for excessive blood loss, and the presence of blood in the pleural space contributes to respiratory insufficiency and infection in this already critically ill subset of patients. Recommended management is prompt drainage of the hemothorax via chest tube with close monitoring of the vital functions and respiratory support if needed. Other options include Video-Assisted Thorascopic Surgery (VATS) decompression for any loculated hemothorax that cannot be adequately managed with chest tube drainage. In fact, our own unpublished institutional experience has demonstrated that late decompression of a hemothorax after TEVAR for rDTAA is associated with both short and long-term poorer outcomes. Hypovolemic shock is a separate predictor [6]. It is a strong predictor of death in the majority of acute aortic syndromes and contributes to increased risk of neurologic deficits as it may lead to inadequate brain and spinal cord perfusion.
Late considerations
Currently, no randomized controlled trials or large prospective studies have compared the outcomes of open and endovascular repair of rDTAA, and the optimal approach for this emergency remains unclear. As time continues and more surgeons continue to use TEVAR, more data will become available. Many questions, though, already have been raised about the long-term durability of stent-graft repair due to the high number of re-interventions associated with its use. Although early peri-operative mortality is decreased with TEVAR, convergence of survival curves of OR v TEVAR is demonstrated over time, suggesting that there may be a trade-off between early survival and late re-interventions leading some to question whether the role of TEVAR may be to postpone death in higher risk patients, and it should be used as a bridging therapy to “get higher risk patients through the initial event of rupture” [6,24]. Desai et al. reported that at 8-10 years the overall survival rate was similar between TEVAR and open repair groups [25].
Despite initial technical success, the main drawback associated with TEVAR is the presence of late complications that may require additional re-intervention. Botsios et al. looked at long-term results of endovascular treatments of non-traumatic ruptured thoracic aortas and detected a high rate of re-interventions, an incidence ranging from 4.5% to 16% after median follow-up of 1.5-44 months [13]. They also emphasize the need for close follow-up over time to ensure that re-interventions, if necessary, can be performed with lower risk in preferably non-urgent settings.
Endoleak
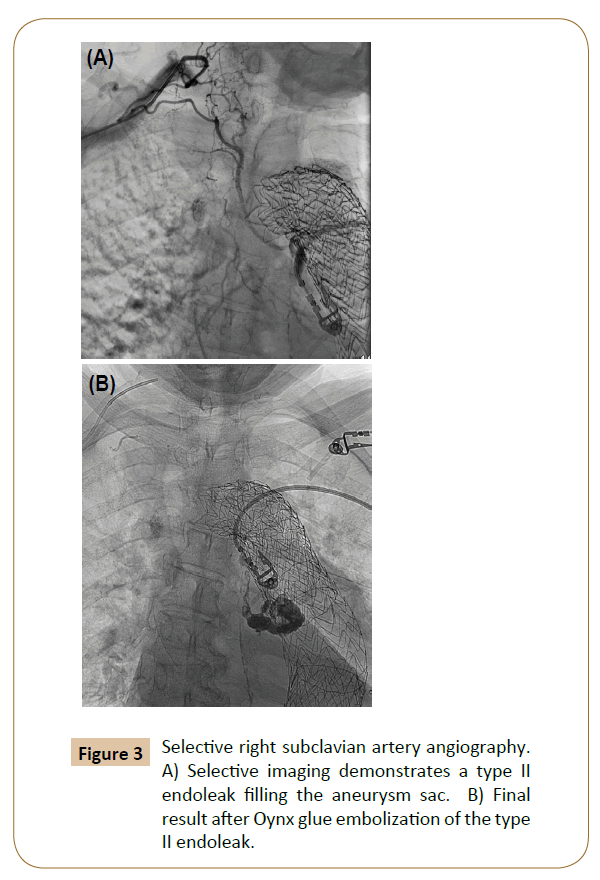
By far one of the most common complications and indications for re-intervention after TEVAR, endoleak is classified based on location. Management generally consists of aggressive endovascular repair when possible of type 1 and 3 endoleaks with observation of type 2 endoleaks. Type 1a endoleaks involve the proximal and type 1b involves the distal landing zones of the stent graft. In patients with degenerative aneurysmal aortic disease, continued aneurysmal degeneration may occur after stent graft repair, which may cause endoleak and sac expansion. For proximal type 1 endoleaks, often more proximal extent coverage is required, which may force coverage of the left subclavian artery. Other more proximal options include extra-anatomic bypass, chimney grafts, or debranching aortic arch procedure in order to preserve blood supply to the aortic arch vessels. Type 2 endoleaks occur from retrograde filling of the aneurysm sac from arteries excluded by the repair--most commonly intercostal arteries and occasionally the left subclavian or bronchial arteries. They can often be observed, however. If sac expansion persists on follow-up, intervention is warranted with embolization (Figure 3). Type 3 endoleaks are the least common and are associated with separation of the modular components of the device or wire frame fractures and associated fabric tears.
Graft infection is one of the rarer yet more challenging complications. Although uncommon, when infection does occur, it may dramatically affect patient outcomes with mortality rates from 18% to 50% [3,25]. Ruptures have higher occurrence of endograft infection, likely due to the urgency of the operation, which may necessitate less than optimal sterile technique [3]. Medical management is attempted if the infection has not compromised aortic integrity or imaging does not show obvious air or fluid around the graft. Open surgical interventions are often required for definitive management [26]. Etiologies include break in sterile technique, bacteremic states, and fistulae to the esophagus or airway [27].
Conclusion
The introduction of TEVAR in 1994 established a new modality in the treatment of aortic pathologies. TEVAR has risen to become the first-line treatment in acute aortic catastrophes. It is clear that TEVAR is associated with early technical success and has become well preferred over open surgical treatment in patients with ruptured thoracic aneurysms. However, due to the rarity of this condition and its emergent nature, it will be very difficult to ever realize a large randomized study comparing the outcomes of TEVAR versus open surgery for rDTAA. Overall, the literature supports management of this aortic catastrophe with TEVAR when it is a suitable option. However, it is recognized that the long-term durability of endovascular repair is poorly established. Long-term surveillance is obviously necessary as late complications are not uncommon and can result in serious consequences if not re-intervened upon appropriately. As more literature emerges regarding the use of these devices for repair of acute aortic pathologies, we can further research long-term outcomes and engender recommendations regarding the role of TEVAR for repair of ruptured thoracic aneurysms.
References
- Dake DM, Miller DC, Semba CP, Mitchell RS, Walker PJ, et al. (1994) Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 331: 1729-1734.
- Coselli JS, Gopaldas RR (2010) Ruptured Thoracic Aneurysms: To Stent or Not to Stent? Circulation 121: 2705-2707.
- Jonker FH, Verhagen HJ, Lin PH, Heijmen RH, Trimarchi S, et al. (2010) Outcomes of endovascular repair of ruptured descending thoracic aneurysms. Circulation 121: 2718-2723.
- Geisbusch P, Kotelis D, Weber TF, Hyhlik-Durr A, Bockler D (2010) Endovascular repair of ruptured thoracic aortic aneurysms is associated with high perioperative morbidity and mortality. J Vasc 51: 299-304.
- Walsh SR, Tang TY, Sadat U, Naik J, Gaunt ME, et al. (2008) Endovascular stenting versus open surgery for thoracic aortic disease: systematic review and meta-analysis of perioperative results. J Vasc Surg 47: 1094-1098.
- Jonker FH, Verhagen HJ, Lin PH, Heijmen RH, Trimarchi S, et al. Open surgery versus endovascular repair of ruptured thoracic aortic aneurysms. J Vasc Surg 53: 1210-1216.
- Goldstein LJ, Ramaiah VG, McKinsey JF (2007) TEVAR for the ruptured thoracic aorta. Endovascular Today 74-78.
- Jonker FH, Trimarchi S, Verhagen HJ, Moll FL, Sumpio BE, et al. (2010) Meta-analysis of open versus endovascular repair for ruptured descending thoracic aneurysm. J Vasc Surg 51: 1026-1032.
- Hoel AW (2013) Aneurysmal disease: Thoracic aorta. Surg Clin North Am 93: 893-910.
- Crawford ES, DeNatale RW (1986) Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg 3: 578-582.
- Girardi LN, Krieger KH, Altorki NK, Mack CA, Lee LY, et al. (2002) Ruptured descending and thoracoabdominal aortic aneurysms. Ann Thorac Surg 74: 1066-1070.
- Dapunt OE, Galla JD, Sadeghi AM, Lansman SL, Mezrow CK, et al. (1994) The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 107: 1323-1332.
- Botsios S, Fromke J, Walterbusch G, Schuermann K, Reinstadler J, et al. (2014) Endovascular treatment for nontraumatic rupture of the descending thoracic aorta: Long-term results. J Card Surg 29: 353-358.
- Hogendoorn W, Schlosser FJ, Muhs BE, Popescu WM (2014) Surgical and anesthetic considerations for the endovascular treatment of ruptured descending thoracic aortic aneurysms. Curr Opin Anaesthesiol 27: 12-20.
- Sayed S, Thompson MM (2005) Endovascular repair of the descending thoracic aorta: evidence for the change in clinical practice. Vascular 13: 148-157.
- Matsumura JS, Lee WA, Mitchell RS, Farber MA, Murad MH, et al. (2009) The Society for Vascular Surgery Practice Guidelines: Management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg 50: 1155-1158.
- Lam CH, Vatakencherry G (2010) Spinal cord protection with a cerebrospinal fluid drain in a patient undergoing thoracic endovascular aortic repair. J Vasc Interv Radiol 21: 1343-1346.
- Scali ST, Wang SK, Feezor RJ, Huber TS, Martin TD, et al. (2014) Preoperative prediction of spinal cord ischemia after thoracic endovascular repair. J Vasc Surg 60: 1481-1490.
- Eskandari MK, Daly CM (2013) Management of late TEVAR failures. In Eskandari MK, Pearce WH, Yao JST, eds. Current Vascular Surgery: 2012. Shelton, CT: People’s medical Publishing House, USA pp: 393-406.
- Jonker FH, Verhagen HJ, Heijmen RH, Lin PH, Trimarchi S, et al. (2011) Endovascular repair of ruptured thoracic aortic aneurysms: predictors of procedure-related stroke. Ann Vasc Surg 25: 3-8.
- Gopaldas RR, Dao TK, LeMaire SA, Huh J, Coselli JS (2011) Endovascular versus open repair of ruptured descending thoracic aortic aneurysms: A nationwide risk-adjusted study of 923 patients. J Thorac Cardiovasc Surg 142: 1010-1018.
- Cambria RP, Crawford RS, Cho JS, Bavaria J, Farber M, et al. (2009) A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg 50: 1255-1264.
- Lee HC, Joo HC, Lee SH, Lee S, Chang BC, et al. (2015) Endovascular repair versus open repair for isolated descending thoracic aortic aneurysm. Yonsei Med J 56: 904-912.
- Amabile P, Rollet G, Vidal V, Collart F, Bartoli JM, et al. (2006) Emergency Treatment of Acute Rupture of the Descending Thoracic Aorta Using Endovascular Stent-Grafts. Ann Vasc Surg 20: 723-730.
- Desai ND, Burtch K, Moser W, Moeller P, Szeto WY, et al. (2012) Long-term comparison of thoracic endovascular aortic repair (TEVAR) to open surgery for the treatment of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 144: 604-609.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, et al. (2011) The relevance of aortic endograft prosthetic infection. J Vasc Surg 54: 327-333.
- Smeds MR, Duncan AA, Harlander-Locke MP, Lawrence PF, Lyden S, et al. (2016) Treatment and outcomes of aortic endograft infection. J Vasc Surg 63: 332-340.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences