Undescribed Anatomical Predictors of Vascular Injury after Fully-Percutaneous Trans Femoral Trans Catheter Aortic Valve Implantation
Javier Tobar, Ignacio J Amat-Santos*, Javier Castrodeza, Irene Martin-Morquecho, Carlos Cortes, Paol Rojas, Hipólito Gutiérrez, Itziar Gomez and Jose A San Román
DOI10.21767/2573-4482.10007
Cardiology Department, Hospital Clínico Universitario de Valladolid, Spain
- *Corresponding Author:
- Ignacio J Amat-Santos
Instituto de Ciencias del Corazón, Hospital Clínico Universitario de Valladolid
Avenida Ramón y Cajal, Valladolid, Spain
Tel: +34983420014
Fax: +34983255305
Email: ijamat@gmail.com
Received date: March 25, 2016; Accepted date: April 19, 2016; Published date: April 27, 2016
Citation: Ignacio J Amat-Santos, et al. Undescribed Anatomical Predictors of Vascular Injury after Fully-Percutaneous Trans Femoral Trans Catheter Aortic Valve Implantation. Journal of Vascular & Endo Surgery. 2016, 1:1. doi: 10.21767/2573-4482.100007
Abstract
Abstract
Background:
Vascular injury (VI) remains frequent after trans catheter aortic valve implantation (TAVI). We aimed to assess the incidence, predictive factors, and the impact of early VI after fully-percutaneous (FP) TAVI.
Method: We included a total of 139 consecutive patients who underwent FP transfemoral TAVI in our institution with 14 to 18Fr sheath systems, through right (119, 85.6%) or left (20, 14.4%) femoral arteries. VI was classified as mayor or minor according to VARC-2 definitions. In hospital data were prospectively collected. Follow-up was available for all patients. Reassessment of femoral artery anatomy as determined by computed tomography was performed including lumen diameters, calcification, tortuosity, height of femoral bifurcation and marked collateral circulation around common femoral artery.
Results: Mean age was 81 ± 6.5, 54% were men, logEuroSCORE were 13.9 ± 7.9 and STS-score was 6.3 ± 4.9. Balloon-expandable and self-expandable devices were used in 14 (10.1%) and 125 patients (89.9%), respectively. Mayor and minor VI were observed in 25 (18%) and in 20 patients (14.3%) respectively, 20 of them due to suboptimal femoral closure (80% of major VI occurring in the first half of the learning curve). Lower platelet count (p=0.043), higher calcification of aortic valve (p=0.049), presence of femoral collaterals (OR=4.5, [95% CI: 1.6-12.9], p=0.005), height of femoral bifurcation (OR=14.5, [95% CI: 5.0-42.1], p<0.001), and failed femoral closure (OR=21.3, [95% CI: 4.5-101.4], p<0.001) were associated to higher rate of VI. The median length of hospitalization was 11.6 days [IQR: 7-14], (15.7 days [IQR: 8-19] in the VI cohort, p<0.001). VI was associated to higher in hospital mortality (13.3 vs. 2.1%, p=0.014).
Conclusion: In patients who underwent FP TAVI, the rate of VI is still high and associated to worse outcomes. A high common femoral artery bifurcation and the presence of collaterals, especially if associated to other concomitant predisposing factors for VI, should be handled with special care and surgical access may be considered.
Keywords
Vascular injury; TAVI; Percutaneous vascular closure; Vascular repair
Introduction
Trans catheter aortic valve implantation (TAVI) is an alternative to surgical aortic valve replacement (AVR) in highrisk patients [1,2]. Over the past years, this procedure has proved to be safe; however there are still related complications that need to be addressed. Probably, the most frequent complication is peripheral vascular injury (VI) and bleeding events involving the access site. According to previous research, major vascular complications during TAVI may range between 5% and 25% of patients [3]. Early VI is often associated to serious bleeding requiring urgent surgical or invasive treatment and, therefore, VI is one of the most frequent causes of in-hospital mortality and, probably, the most worrisome safety issue during fully percutaneous procedures. Moreover, late survival rate seems to be also conditioned by early VI according to the long-term published results of the PARTNER trial [4,5]. Complications in the vascular access site are probably influenced by several factors, including technical issues as the size of the devices (progressively improving due to lower profile of newer generation devices), the learning curve of fully-percutaneous procedures, and also, factors related to patient´s anatomy [6]. Our aims were: 1) To evaluate the incidence of early VI and to define the clinical and anatomical predictors through computed tomography (CT) analysis including a detailed description of femoral-iliac axis particularities, and 2) To assess the impact of early VI on post-TAVI prognosis.
Methods
Study population
Single-center, observational study of early VI related to TAVI. We collected the data of 139 consecutive patients who underwent TAVI in our center between April, 2009 and October, 2015. TAVI was decided by the local Heart Team following the recommendations of the European Society of Cardiology (in high risk patients) or when there were other criteria that precluded from conventional surgery despite lower risk score (including porcelain aorta and liver disease).
Procedural methods
The procedures were performed through 14Fr to 18Fr sheath systems, using right (119, 85.6%) or left (20, 14.4%) femoral arteries on the basis of CT angiography parameters that included, minimal lumen diameter, degree of calcification, and severity of tortuosity. The vascular access was achieved in all cases percutaneously with pre-closure technique based on the puncture of the common femoral artery defined by contralateral ilio-femoral angiography. A single Prostar® or ProGlide® (Abbott Vascular, Santa Clara, CA, USA) device was placed in femoral artery before the aortic valve deployment. After tightening the sutures, a final angiogram was performed from contralateral side. The puncture was not ultrasoundguided.
Data gathering
All in hospital data were prospectively collected. Follow-up was available for all patients through clinical visit at 1 month, 6 month and 1 year after discharge.
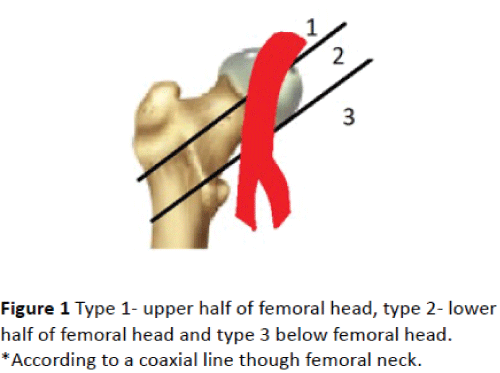
Re-assessment of the femoral arteries’ anatomy was determined by re-evaluation of the computed tomography images by 2 independent operators (J. T. and I. J. A. S) that determined: 1) minimal diameter of the access site. Vessel diameter was defined as the distance between the internal vessel walls. The diameters of the common femoral arteries were measured below the inguinal ligament. 2) Femoral artery severe wall calcification, defined as the presence of calcium >50% of the arterial perimeter (“C” shape) or bulky calcification protruding to the lumen of the vessel. 3) Presence of marked collateral circulation (defined as 3 or more collateral vessels around common femoral artery visualized with angiography). 4) Height of the bifurcation of common femoral artery, determined as schematically depicted in Figure 1.
The assessment of vascular injury was determined by 2 independent operators (J.T. and I.J.A.S); in case of discrepancy a third observer (J.C.) contributed to the final decision. Vascular injury major or minor was defined according to the consensus from the Valve Academic Research Consortium (version 2) [7]. The study group was divided into two subgroups to assess the influence of a learning curve on early vascular injury. Subgroups presented consecutive patients that underwent TAVI earlier and later during the follow-up period.
Statistical methods
The data are expressed as absolute rate and percentage in case of qualitative variables. Quantitative variables are described as mean (SD) or median (25th-75th) interquartile range [IQR]) depending on variable distribution. Group comparisons were analyzed using Student’s t-test or its nonparametric equivalent, Mann-Whitney U-test for continuous variables, and Chi-square test or Fisher’s exact test for categorical variables. Statistical significance was defined as pvalue <0.05. The univariate normality assumptions were verified with the Kolmogorov-Smirnov tests. A cox multivariable analysis including all variables with P value <0, 10 in the univariable analysis was used to determinate the predictive factors of vascular injury. All analyses were conducted using the statistical package SPSS, version 18.0 (SPSS, Inc.; Chicago, Illinois, USA).
Results
A total of 139 consecutive patients (pts) were included. Mean age was 81 ± 6.5, 54% were men, logistic Euroscore was 13.9 ± 7.9 and STS-score was 6.3 ± 4.9. The balloonexpandable SAPIEN XT/SAPIEN-3 and the self-expandable Medtronic Core Valve were used in 14 (10,1%) and 125 patients (89,9%), respectively. Mayor and minor VI were observed in 25 (18%) and in 20 patients (14.3%) respectively, 20 of them due to suboptimal femoral closure. The different types of VI, the severity, and their treatment are briefly described in the Table 1. The learning curve had impact on early VI rate after TAVI, with 80% of the episodes of major VI occurring in the first half of our population.
| Type of CV event | All VI events n=45 | Mayor VI n=25 | Minor VI n=20 | Intervention |
|---|---|---|---|---|
| Retroperitoneal bleeding | 5 | 5 | 0 | Vascular surgery -3 Death -2 Transfusion - 1 |
| Aorta rupture | 2 | 2 | 0 | Death- 2 |
| Femoral artery rupture | 1 | 1 | 0 | Femoral stenting |
| Femoral artery dissection | 4 | 1 | 3 | Surgery - 1 Femoral stenting -1 No intervention- 2 |
| Femoral stenosis/occlusion | 4 | 3 | 1 | Vascular surgery - 3 No intervention |
| Closure failure | 12 | 7 | 5 | Vascular surgery - 8 Balloon stenting- 4 |
| Femoral distal embolization | 2 | 2 | 0 | Vascular surgery -2 |
| Hematoma | 5 | 0 | 5 | Blood transfusion - 5 |
| Femoral pseudo aneurysm | 8 | 4 | 4 | Surgery -4 Compression -4 |
| Femoral arterio-venous fistulae | 2 | 1 | 1 | Surgery -1 Compression -1 |
CV: Cardiovascular; VI: Vascular Injury
Table 1: Description of the different types of vascular injury and their treatment.
Main predictors of VI are summarized in Table 2. To remark lower platelet count (173 vs. 192 × 10³, p=0.043), higher calcification of aortic valve (3692 UA vs. 2486 UA, p=0.049), the presence of collateral circulation (P=0.023), and height of femoral bifurcation (type 1 vs. types 2 and 3 according to Figure 1) (p<0.001) were related to higher rate of VI. The 62 patients (44.6%) who presented a height of the common femoral artery bifurcation at the level of the femoral head or above (types 1 and 2 in Figure 1) suffered VI in up to 82,2% of the cases, as opposed to 17.8% (8 patients) among those with bifurcation type 3 (77 patients, 55.4%), p<0.001.
| Total of patients n=139 | Without VI (%) n=94 patients | With VI (%) n=45 patients | P value | |
|---|---|---|---|---|
| Age (years) | 81.01 +/-6.5 | 81.48 +/-6.1 | 80 +/- 7.1 | P=0.216 |
| Female | 64 (46%) | 40 (42.6%) | 24 (53.3%) | P=0.233 |
| Diabetes mellitus | 49 (35.3%) | 31 (33%) | 18 (40%) | P=0.418 |
| Hypertension | 45 (32.4%) | 29 (30.9%) | 16 (35.6%) | P=0.579 |
| Dyslipidemia | 53 (38.1%) | 36 (38.3%) | 17 (37.8%) | P=0.953 |
| Peripheral vascular disease | 8 (5.8%) | 5 (5.3%) | 3 (6.7%) | P=0.71 |
| Stroke/TIA | 13 (9.4%) | 9 (9.6%) | 4 (8.9%) | P=0.99 |
| AR (3 and 4 degree) | 16 (11.6%) | 9 (9.6%) | 7 (15.9%) | P=0.279 |
| Body mass index (kg/m2) | 26.87 +/- 4.6 | 27.2 +/- 4.6 | 26 +/- 4.6 | P=0.164 |
| Hemoglobin (g/dL) | 11.9 +/- 1.9 | 11.84 +/-5.6 | 12 +/-5.9 | P=0.614 |
| Platelets (mil/mm3) | 179 +/-53 X 10³ | 192 +/- 48 X 10³ | 172 +/-59 X 10³ | P=0.043 |
| Serum creatinine (mg/dL) | 1.11 +/- 0.4 | 1.15 +/- 0.43 | 1.04 +/- 0.31 | P=0.104 |
| Coronary artery disease | 62 (44.7%) | 42 (44.7%) | 20 (44.4%) | P=0.979 |
| Porcelain aorta | 9 (6.5%) | 4 (4.3%) | 5 (11.1%) | P=0.149 |
| Right femoral approach | 119 (85.6%) | 82 (87.2%) | 37 (82.2%) | P=0.431 |
| Collateral circulation | 46 (33.1%) | 25 (27.2%) | 21 (46.7%) | P=0.023 |
| STS score (%) | 6.34 +/- 4.9 | 6.59 +/- 4.7 | 5.82 +/- 5.3 | P=0.07 |
| LogEuroSCORE | 13.89 +/-7.9 | 13.15 +/-8.9 | 15.44 +/- 8.6 | P=0.292 |
| Bifurcation femoral | ||||
| Type 1 | 9 (6.5%) | 1 (1.1%) | 8 (17.8%) | P=0.001 |
| Type 2 | 53 (38.1%) | 24 (25.5%) | 29 (64.4%) | |
| Type 3 | 77 (55.4%) | 69 (73.3%) | 8 (17.8%) | |
| Prostar® closure device | 114 (83.8%) | 78 (83.9%) | 36 (83.7%) | P=0.982 |
| Severe femoral wall calcification | 31 (22.5%) | 19 (20.2%) | 12 (27.3%) | P=0.354 |
| Self-expandable Valve | 125(89.9%) | 85 (90.4%) | 40 (88.9%) | P=0.946 |
| Minor femoral diameter* | 7.42 +/-1.31 | 7.57 +/- 1.28 | 7.07 +/- 1.32 | P=0.069 |
AR: Aortic Regurgitation; TIA: Transient Ischemic Accident *(mm)
Table 2: Baseline and procedural characteristics of the patients. Main predictors of vascular injury in our study population.
Concerning the procedure, the use of Prostar® device (n=114, 83.8%) for access site closure or ProGlide® system (n=22, 16.2%) (Abbott Vascular Inc., Santa Clara, CA, USA) was not associated to differences in outcomes in terms of VI. Neither was the type of prosthesis implanted as shown in Table 2.
The median length of hospitalization was 11.6 days [IQR: 7-14], being longer for patients who complicated with VI (15.7 days [IQR: 8-19), p<0.001). The in-hospital rates of acute kidney injury, heart failure, and need for oro-tracheal intubation were higher in patients who suffered major vascular injury (p<0.001).
Multivariate analyses helped to determine that height of bifurcation (OR=14.5, [95% CI: 5.0-42.1], p<0.001), presence of collateral circulation (OR=4.5, [95% CI: 1.6-12.9], p<0.001), and failed femoral closure (OR=21.3, [95% CI: 4.5-101.4], p<0.001) were independent predictors of VI. Patients who presented in hospital death (8 patients, 5.8%), also had a higher rate of VI (75% vs. 25%, p=0.014). Post-procedural VI was the main reason for 4 early deaths (within the first 24 h after the implantation), 2 of them due to retroperitoneal bleeding and 2 due to aortic rupture. The other 4 patients died due to septic shock by nosocomial pneumonia in 2 cases and heart failure in the remaining 2 cases.
The impact of VI during the hospitalization did no significantly impact the outcomes at 1year of follow up.
Discussion
According to previous data, vascular injury after transfemoral TAVI has raised as the most common peri-procedural complication and with the worst effect on outcomes [8]. Our results highlight the impact of this problem in the in-hospital mortality rate despite a significant decrease of this complication together with the progression of the learning curve and the better profile of newer devices. However, the impact of large semi-rigid introducers in severely atherosclerotic peripheral vessels is still a challenge that requires exquisite evaluation of the anatomy. To this end, the lessons learnt with the experience of CT analysis should not be denied. Beyond vessels diameter and calcification, severalfactors including tortuosity, presence of a prominent collateral circulation around common femoral artery, and a bifurcation of the access site in the upper segment of the femoral head have demonstrated in this study to act as predictors of vascular complications. Importantly, the development of VI was associated to higher in-hospital mortality, suggesting that the presence of adverse femoral anatomy (even if diameters are adequate) should lead to switch to surgical cut-down or, at least, promote the presence of the surgeon during the procedure in order to promptly correct eventual vascular damage by the TAVI system. The higher rate of VI reported in series with fully percutaneous approach as compared to those with surgical cut-down [5-8] has been a source of conflict. Although surgical cut-down can be performed with local anesthesia, in many centers general anesthesia is still used for such intervention. The current tendency to minimize the complexity of the procedures includes the avoidance of oro-tracheal intubation and favors the use of fully percutaneous approach due to the general belief that this strategy may improve outcomes in frail patients. However, as lower risk patients undergo this procedure more and more often, competitive results to those of open heart surgery can only be obtained through optimization of outcomes with reduction of main complications as VI [8,9]. In addition, the impact of the learning curve is probably more relevant in fully percutaneous procedures as highlights the fact that 80% of the major vascular complications in our population occurred in the first half of the treated patients.
With regard to arterial morphology, we demonstrated that a greater arterial wallcalcification, height of bifurcation, and presence of collateral circulation significantly increased the risk for early VI. Moreover, collateral circulation and especially height of bifurcation were independent predictors of early VI. Collaterals may reflect a worse vascular disease, with stenotic peripheral vessels that promote the development of such collaterals. But also, it may be the source of unnoticed punction of these vessels that may bleed in the peri-procedural period. The impact of the height of the bifurcation can probably be explained by the fact that, this height also reflects the need for a deeperfemoral punction. Even when this maneuver is normally angiographically guided, as the tip of the needle is deeper, control of its trajectory is worse. Also, the knots of the percutaneous closure devices had higher risk of tighten too far from the vessels’ wall, not achieving a correct hemostasis.
Previous research had tried to address the worrisome issue of VI through real-time ultrasound guidance. Compared with standard fluoroscopic guidance, they achieved to improve common femoral artery cannulation only in the subgroup of patients with higher bifurcations [10]. Although surgical cut-down has been supported by some groups, as experience with percutaneous approach increases, this less invasive percutaneous method seems associated with similar rates of major and minor vascular complications, with lower access site infection and bleeding, and shorter hospital stay compared to the surgical approach [11]. Nevertheless, a role to surgical vascular approach may continue to be necessary in patients that present a combination of anatomical predisposing factors for VI, as previously described.
The main limitation of our study was its retrospective nature and single-centerregistry of data. However, report of this real-world experience is of utmost importance in order to draw a picture of outcomes and main complications in moderate-volume centers so that the best strategies to improve the results can be implemented, mainly, as lower risk patients are the new target for this technology.
Conclusion
In conclusion, in spite of the careful patient-level selection in order to avoid vascular access complications, vessels’ injury can still occur, being likely related to a higher rate of inhospital mortality. More detailed anatomical description of vascular access will be required in the future in order to improve outcomes. Also, surgical cut-down, should not be excluded in case of extremely challenging anatomies.
References
- Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, et al. (2007) Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 116: 755-763.
- Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, et al. (2006) Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am CollCardiol 47: 1214-1223.
- Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, et al. (2012) Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted metaanalysis of 3,519 patients from 16 studies. J Am CollCardiol 59: 2317-2326.
- Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, et al. (2012) Two-year outcomes after transcatheter or surgical aortic-valve replacement. New Engl J Med 366: 1686-1695.
- Hanzel GS, Harrity PJ, Schreiber TL, O’Neill WW (2005) Retrograde percutaneous aortic valve implantation for critical aortic stenosis. Catheter CardiovascInterv. 64: 322-326.
- Van MieghemNM, Tchetche D, Chieffo A, Dumonteil N, Messika-Zeitoun D,et al. (2012) Incidence, predictors, and implications of access site complications with transfemoraltranscatheter aortic valve implantation. Am J Cardiol 110: 1361-1367.
- Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, et al (2012) Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. Eur Heart J 33:2403–2418ion. Am J Cardiol 110: 1361-1367.
- Hayashida K, Lefevre T, Chevalier B, Hovasse T, Romano M, et al (2011) Transfemoral aortic valve implantation new criteria to predict vascular complications. J Am CollCardiolIntv 4: 851–858.
- Eltchaninoff H, Durand E, Borz B, Godin M, Tron C, et al (2012) Prospective analysis of 30 day safety and performance of transfemoraltranscatheter aortic valve implantation with Edwards SAPIEN XT versus SAPIEN prostheses. Arch Cardiovasc Dis 105: 132–140.
- Seto AH, Abu-Fadel MS, Sparling JM, Zacharias SJ, Daly TS, et al. (2010) Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial) JACC CardiovascInterv 3: 751–758.
- Holper EM, Kim RJ, Mack M, Brown D, Brinkman W (2014) Randomized trial of surgical cutdown versus percutaneous access in transfemoral TAVR. Catheter CardiovascInterv 83: 457–464.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences